Improving COVID Patient Outcomes with Blood Thinners
"Early in the pandemic, physicians around the world observed increased rates of blood clots and inflammation among COVID-19 patients which affected multiple organs and led to complications such as lung failure, heart attack and stroke.""Whether providing increased doses of blood thinners routinely administered to hospitalized patients would be safe and effective was unknown at that time."National Institutes of Health, U.S."With the ICU beds being under so much pressure, this is a medication we have that can reduce the need for patients to go to the ICU.""It is great news."Dr.Lana Castellucci, associate scientist, Ottawa Hospital Research Institute
 |
A new study with global participation encompassing no fewer than 300 health centres around the world has concluded that administering full doses of blood thinners to patients hospitalized and placed in intensive care units (ICUs) reduced the need for ventilators and led to improved outcomes. Among the participating centres was the Ottawa Hospital where thrombosis physician Dr.Lana Castellucci led the study's Ottawa arm.
The purpose of the study was to more fully understand preventive therapies that could alleviate the harm produced by unusual blood clotting, a marker of COVID-19 which can go on to cause significant organ damage. The study found patients given the full dose of blood thinner ended up requiring less vital organ support, including ventilation. A possible reduction in deaths resulting from the treatment was also suggested by the study results.
 |
| (Jeffrey Sauger/Bloomberg/Getty) |
As well as improving outcomes for some patients with COVID-19, the treatment would have the added effect of removing pressure from hospital intensive care units. A low dose of the blood thinner heparin was already routinely being administered at the Ottawa Hospital to patients admitted and eligible, for the purpose of reducing blood clot risk. Hospitalization itself is considered a risk factor.
That a higher dose of blood thinners administered to COVID patients has such a beneficial effect on outcome represents a substantial bonus both for patients and the health care system, reason enough to laud the study and its results. Dr. Ewan Goligher, a co-chair on one of the clinical trials stated a sense of relief, given that many people worldwide end up in intensive care as a result of contracting COVID-19.
"They're very, very ill, they're often in the ICU for a long time. It's a devastating life event. Even if they do survive, it means immense suffering, and to prevent people from becoming critically ill is huge."Dr.Ewan Goligher, critical care physician, Toronto General Hospital
 |
| A health-care worker pauses during a lull in visitors at a COVID-19 testing centre in Toronto. THE CANADIAN PRESS/Chris Young |
"In large clinical trial conducted worldwide, full dose anti-coagulation (blood thinner) treatments given to moderately ill patients hospitalized for COVID-19 reduced the requirement of vital organ support—such as the need for ventilation. A trend in possible reduction of mortality was also observed and is being further studied. With large numbers of COVID-19 patients requiring hospitalization, these outcomes could also help reduce the overload on intensive care units around the world."Based on the interim results of more than 1,000 moderately ill patients admitted to hospital, findings showed that full doses of blood thinners, in addition to being safe, were superior to the doses normally given to prevent blood clots in hospitalized patients—with regard to the primary endpoint which is the need for ventilation or other organ supportive interventions. The trial investigators are now working as fast as possible to make the full results of the study available so clinicians can make informed decisions about treating their COVID-19 patients."As is normal for clinical trials, these trials are overseen by independent boards that routinely review the data and are composed of experts in ethics, biostatistics, clinical trials, and blood clotting disorders. Informed by the deliberations of these oversight boards, all the trial sites have stopped enrollment."However, research questions remain about how to further improve the clinical care of COVID-19 patients. This adaptive protocol has been designed to allow different drugs to be started, stopped or combined during the study in response to emerging scientific data. This approach enables the rapid testing of additional agents without compromising safety and the study will evolve accordingly."National Institutes of Health
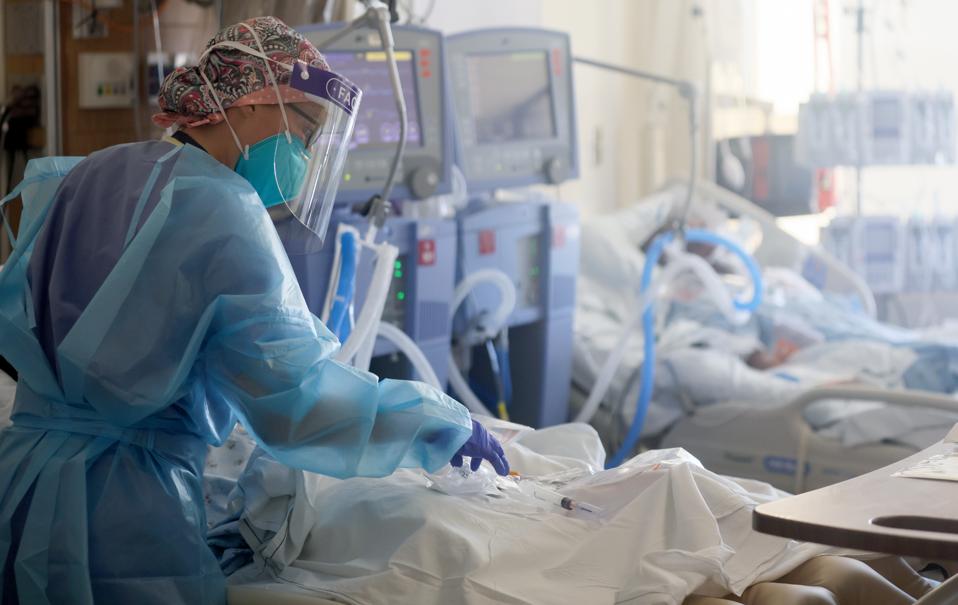 |
TORRANCE, CALIFORNIA - JANUARY 21: U.S. Air Force 1st Lt. Allyson Black Getty Images |
Labels: Blood Thinners, COVID-19, Therapeutic Study

0 Comments:
Post a Comment
<< Home