Moving Ahead with CRISPR Therapy
"This is the first step toward the use of gene editing therapies to treat a wide variety of rare genetic disorders for which there are currently no definitive medical treatments.""As we get better and better at making these therapies and shorten the time frame even more, economies of scale will kick in and I would expect the costs to come down.""Think of it like a GPS signal. You can change where the GPS is going depending on what specific sequence of genes you want to change.""We want each and every patient to have the potential to experience the same results we saw in this first patient, and we hope that other academic investigators will replicate this method for many rare diseases and give many patients a fair shot at living a healthy life.""The promise of gene therapy that we’ve heard about for decades is coming to fruition, and it’s going to utterly transform the way we approach medicine."Dr. Kiran Musunuru, gene editing expert, University of Pennsylvania"Years and years of progress in gene editing and collaboration between researchers and clinicians made this moment possible, and while KJ is just one patient, we hope he is the first of many to benefit from a methodology that can be scaled to fit an individual patient’s needs.""While KJ will need to be monitored carefully for the rest of his life, our initial findings are quite promising."Rebecca Ahrens-Nicklas, MD, PhD, director, Gene Therapy for Inherited Metabolic Disorders Frontier Program (GTIMD), Children’s Hospital of Philadelphia
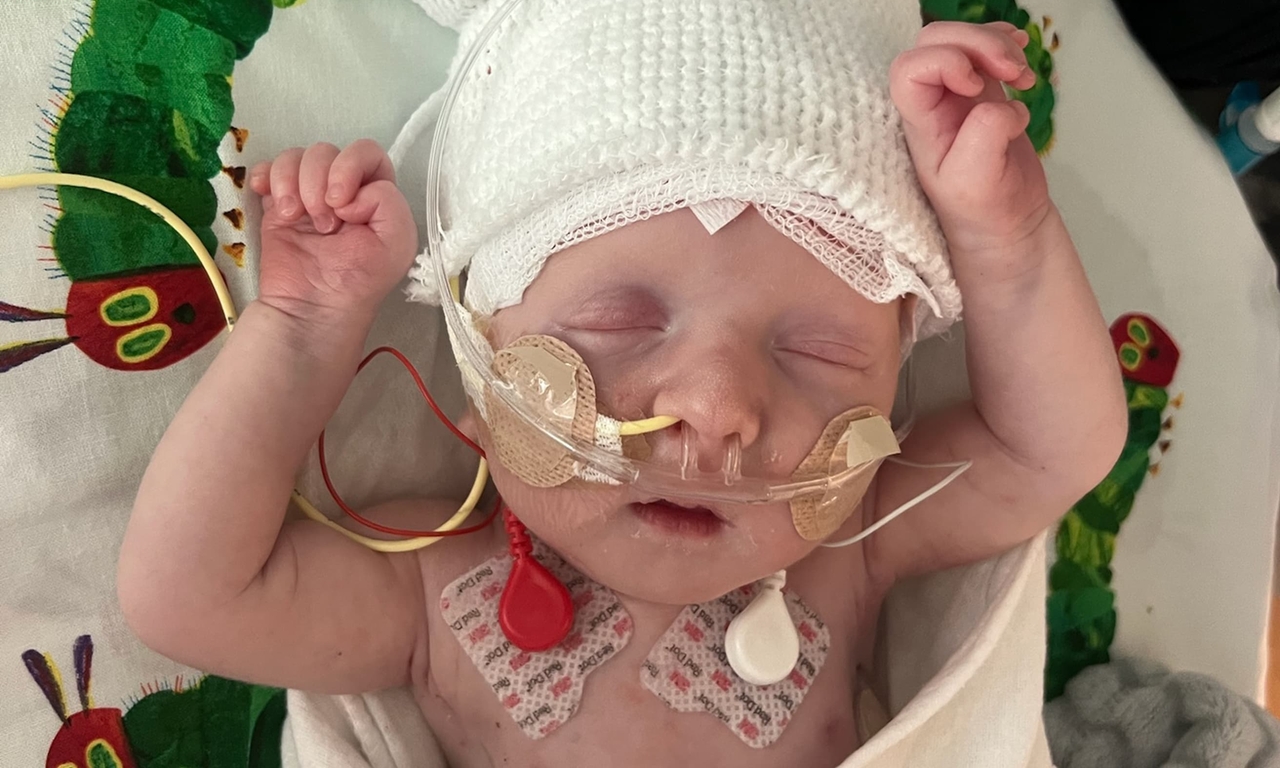 |
| KJ was only days old when he was diagnosed with a rare metabolic disorder and transferred to Children's Hospital of Philadelphia where doctors were actively researching new cell and gene therapies. |
"[We weighed] all the options, asking all the questions for either the liver transplant, which is invasive, or something that's never been done before.""We prayed, we talked to people, we gathered information, and we eventually decided that this was the way we were going to go.""[Considering his poor prognosis earlier], any time we see even the smallest milestone that he's meeting -- like a little wave or rolling over -- that's a big moment for us.""We would do anything for our kids, so with KJ, we wanted to figure out how we were going to support him and how we were going to get him to the point where he can do all the things a normal kid should be able to do.""We thought it was our responsibility to help our child, so when the doctors came to us with their idea, we put our trust in them in the hopes that it could help not just KJ but other families in our position."Kyle and Nicole Muldoon, parents of infant KJ Muldoon
 |
| Kiran Musunru, MD, PhD, MPH, ML, MRA, (left) and Rebecca Ahrens-Nicklas, MD, PhD, (right) led the group of researchers from CHOP and Penn who developed a personalized treatment for baby KJ. |
A new study has been published, describing by researchers an experimental therapy highlighting a baby born with a rare and dangerous genetic disease who is now growing and thriving following a gene editing treatment designed specifically for him. The study was published in the New England Journal of Medicine. The child is among the first ever to be treated successfully with a custom therapy meant to correct a tiny, critical error on his genetic code, one that kills fifty percent of affected infants.
It is the hope of doctors that some day in the near future emerging technology can help millions of people whose conditions are rare -- often referred to as 'orphan' conditions -- as genetic medicine advances. The era awaits when similar personalized treatments become available for individuals afflicted with rare conditions. The baby, identified as KJ Muldoon of Clifton Heights, Pennsylvania represents one of some 350 million individuals worldwide with mostly genetically inherited rare diseases.
Infant KJ was diagnosed soon after his birth with severe CPS1 deficiency which according to experts affects about one in a million babies who lack an enzyme required to help remove ammonia from their body. Their condition can lead to the ammonia building up in their blood to become toxic. For some, a liver transplant is an option. For baby KJ, once his parents had agreed, the team at Children's Hospital of Philadelphia and Penn Medicine created an individualized therapy to correct the child's faulty gene.
CRISPR, the gene editing tool representing a huge step forward in understanding nature's architectural designs of human biology, led to researchers using a technique to flip the mutated DNA 'letter' -- known as a base -- to its correct version. "Base editing" reduces risk of unintended genetic changes. KJ received his initial IV infusion in February, when the gene editing therapy was delivered through tiny fatty droplets named lipid nanoparticles, absorbed by liver cells.
March and April saw follow-up doses, the therapy enabling KJ to consume food more normally. He was able to recover well from illnesses such as colds which can exacerbate symptoms of CPS1. Now 9-1/2 months old, the infant currently requires reduced medication. The hope of the researchers involved is that what they learn from treating KJ will assist in more fully understanding other rare disease patient condiions.
Generally, more common disorders are targeted by expensive-to-develop gene therapies afflicting much greater numbers of people. For simple financial reasons a greater number of patients equate with greater sales, the profits of which can help with development costs and generate greater profit. Sickle cell disease, a blood disorder that affects millions of people globally saw the first CRISPR therapy approved by the U.S. Food and Drug administration.
"Once someone comes with a breakthrough like this, it will take no time [for other teams to apply the lessons and move forward].""There are barriers, but I predict that they are going to be crossed in the next five to ten years.""Then the whole field will move as a block because we're pretty much ready."Carlos Moraes, neurology professor, University of Miami
 |
| KJ's parents, Kyle and Nicole, and his three siblings are looking forward to welcoming him home after a first-of-its-kind personalized gene editing therapy at CHOP. Children's Hospital of Philadelphia |
Labels: Anticipating Wider Use Within the Medical Community. Gene Editing, CRISPR Therapy, Individually Designed Approaches to Rare Conditions, Rare Diseases

0 Comments:
Post a Comment
<< Home