Human Guinea Pigs? Challenge Trials; SARS-CoV-2
"Good for Britain, doing challenge trails over the all too predictable bleating of some bioethicists.""In my view, taking a known risk can be done ethically. The 'first, do no harm' principle doesn't necessarily apply here.""Trials are experimentation. They're not intended with the same goal as routine treatment in medicine.""With firefighters [as an analogy] you recruit people who are willing to take the risk to run into a burning building. They know that running into a burning building can kill them, they know there may be no rescue for them. But they nonetheless volunteer to do it, for high stakes, surely, although less high than a global pandemic, which is on its way to killing millions."Dr.Amir Attaran, professor of law and medicine, University of Ottawa"We do feel that political leaders should come forward, because we would like Canada to be part of the solution, rather than waiting for others to do this. We do allow people to volunteer for things that carry risk. People can join the military during war because they want to help their country, and we don't say that's unethical because they might get harmed.""We don't know how well any of these [late-stage human trial vaccines] are going to work -- unless we are exploring all options, there is a strong chance that it takes longer and longer before we have a solution.""That's more deaths, more people getting sick, more people spending more time in ICU, hospitals having to reduce surgeries because we have too many people ill and too many people living in isolation."Dr.Michael Silverman, chief, division of infectious diseases, Schulich School of Medicine, Western University
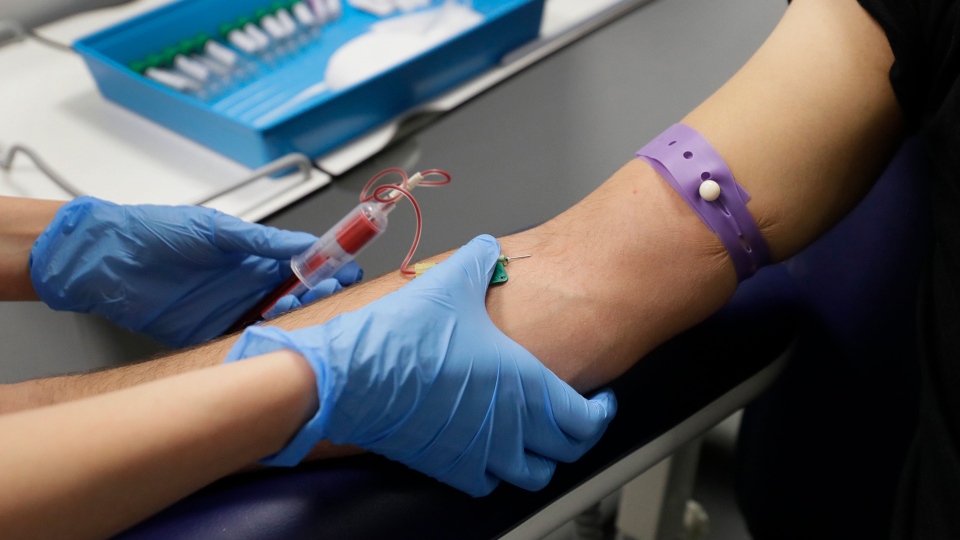 |
| Clinical Research Nurse Aneta Gupta takes bloods from volunteer Yash during the Imperial College vaccine trial at a clinic in London, Wednesday, Aug. 5, 2020. Scientists at Imperial College London are immunizing hundreds of people with an experimental coronavirus vaccine in an early trial after seeing no worrying safety problems in a small number vaccinated so far. (AP Photo/Kirsty Wigglesworth) |
An e-petition addressed to Parliament went into circulation, co-sponsored by Canadian Member of Parliament Marcus Powlowski from Thunder Bay, Ontario and co-signed by a number of Canadian infectious diseases experts. It asks Parliament to urgently and publicly give official support to a plan that would permit scientists intentionally infecting people with the SARS-CoV-2 virus that leads to COVID-19, a deadly scourge ravaging all countries of the global community. This, in a serious, time-sensitive move to help in the discovery of a coronavirus-effective vaccine.
The e-petition reads in part: "The risk to young, healthy volunteers is on par with other acts of public service, such as living kidney donations". The point is to achieve popular support of human challenge trials for COVID-19 in Canada, emulating experiments in preparation to take place at the Royal Free Hospital in London, England, announced this week by the British government. Their active participation in backing human challenge trials led by Imperial College London scientists together with a subsidiary of a Dublin-based medical research company appeals to the 600 individuals who signed the e-petition in Canada.
Under the coordinated British scenario the initial tranche of volunteers are to enter a 19-bed isolation facility early in January where they will be infected with a purified strain of the live virus that would be introduced into their noses for deliberate infection. The intention is that the researchers' first order of business is to ascertain a minimum dose of the pathogen required for an active infection through introducing first tiny amounts of virus, increasing the dose as required to the point where it is clear the person develops mild COVID symptoms.
At which point researchers plan to inject volunteers with test vaccines, exposing them to the challenge dose in hopes of understanding how well the vaccines proffer the needed protection. That there is no rescue therapy available for this challenge trial fails to sit well with some within the medical-scientific community. Recently the warning of the "twin risks of exploitation and coersion" of volunteers was voiced by Dalhousie University philosopher Francoise Baylis and bioethicist Landon Gertz.
"Volunteers will be quarantined and compensated, likely around $6,500+ [the current rate of remuneration for influenza challenge studies in Britain], but possibly higher. Is $6,500 too little or too much for those consenting to a small chance of death or serious disability? What is fair compensation for agreeing to be infected with a deadly pathogen and then quarantined?"
Close to 39,000 would-be volunteers from across the world inclusive of 1,500 Canadians, expressed their interest in being part of a human challenge test, contacting the advocacy group 1Day Sooner. Young and healthy 18 to 25-year-olds with no underlying illnesses are considered to be at low risk of death from COVID-19, and it is from among that demographic that the researchers have chosen to select volunteer subjects.
The idea behind the human challenge trial is to cut waiting time and thus, in the long range, save lives with an expedited vaccine based partly on the conclusions reached through the challenge trial results. Ordinarily it takes longer to determine whether a vaccine is working when it is used in a scenario where virus circulation within a community is at low ebb. In comparison, "challenge studies take much less time, require fewer volunteers, aren't tethered to natural infection rates and can therefore accelerate progress", explained medical ethicists Kyle Ferguson and Arthur Caplan of NYU Grossman School of Medicine.
Nature journal has reported that volunteers are to be treated with antiviral drugs like remdesivir immediately a nurse swab tests positive for the SARS-CoV-2 virus. World Health Organization interim study results had concluded that remdesivir results identified itself as no more effective than a placebo at limiting the need for mechanical ventilation or the risk of death in severely ill patients with COVID.
 |
"It takes a LONG time to get these trials going [challenge trials].""[Debate rages about rescue treatment; we don't have one.] As such, are we really justified in intentionally infecting healthy human volunteers -- even those that fully appreciate what they are consenting to, and are at low risk for complications and death from COVID-19 -- given that we are still discovering much about SARS-CoV-2 and its long term impact?""I just don't think we've hit the bar to justify the risk -- we may get there, but I don't think we are there now."Claudia Emerson, philosopher, McMaster University
Labels: Canada, Challenge Trials, Research, SARS-CoV-2, United Kingdom, Vaccines

0 Comments:
Post a Comment
<< Home