Enlargening the Medical Arsenal Against COVID
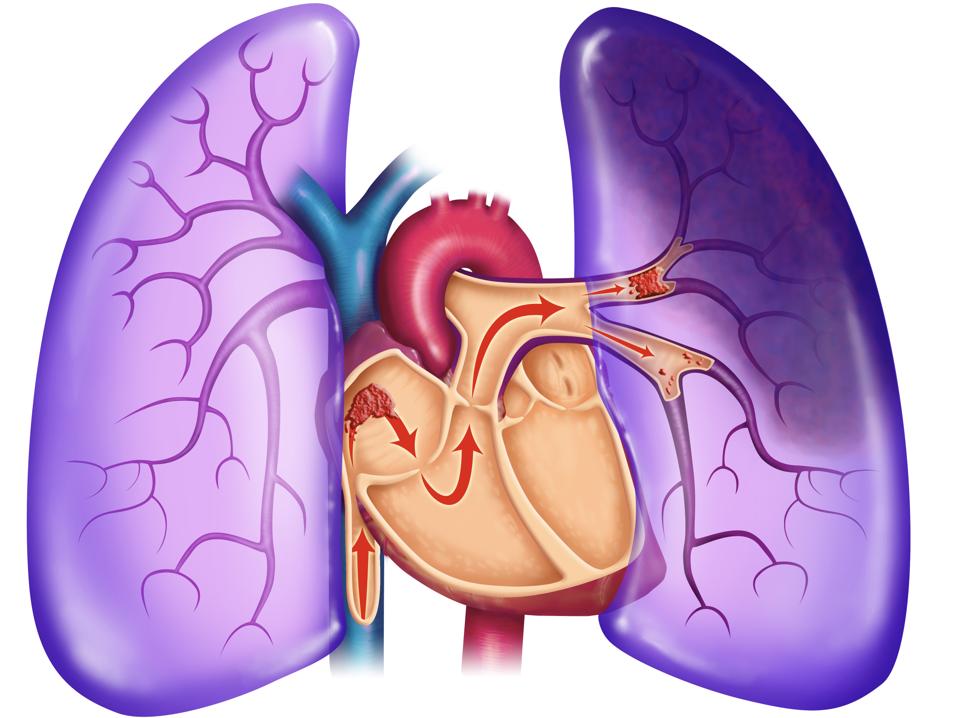 |
Pulmonary Embolism Universal Images Group |
"Aspirin is a very widely available, cheap drug, and if it were to work, that would be a huge treatment boost. Anti-clotting drugs is an area where we did have a gap.""You can end up several months down the line with tens of thousands of people having taken a drug and we still don't know if it worked.""In the U.S. they have given tens of thousands of patients convalescent plasma outside trials. If they had put them in a trial we would know if it worked by now."Professor Peter Horby, Oxford University, chief investigator, Recovery trial
The Recovery trial, out of Oxford University in Great Britain, represents a team of scientists led by Professor Horby, investigating a number of drugs and therapeutic treatments related to SARS-CoV-2, including scheduling an investigation of the efficacy of trial results into convalescent plasma. This is blood plasma comprised of blood of survivors of COVID-19 which contains powerful antibodies. In Britain the largest convalescent plasma trial in he world is being run with three thousand patients signed up to participate, half to be given the treatment and the remainder to act as a control arm, with a placebo in place of the convalescent plasma.
Professor Horby's tart observation represents an oblique criticism of the failure to act on the crucial tool that trials represent in shedding light on what works and what doesn't. He is critical of the practise of merely doling out speculative drugs to patients. He was addressing the science and technology select committee, informing them of his team's progress on the Recovery trial, introducing them to a new concept in the treatment of COVID-19, with anti-clotting medication. In this instance, common Aspirin, used conventionally with heart patients in low dose daily therapy to avoid blood clotting.
In a new trial to be speedily initiated, COVID-19 patients admitted to British hospitals will be prescribed Aspirin (acetylsalicylic acid (ASA) in an effort to discover whether the drug could prevent deadly blood clotting in the lungs. A number of studies have shown that close to 80 percent of people who die of COVID-19 develop thrombosis -- blood clotting -- in the lungs. A condition that prevents oxygen from moving through the body and generally speaking, in most circumstances when left undetected and untreated can be fatal.
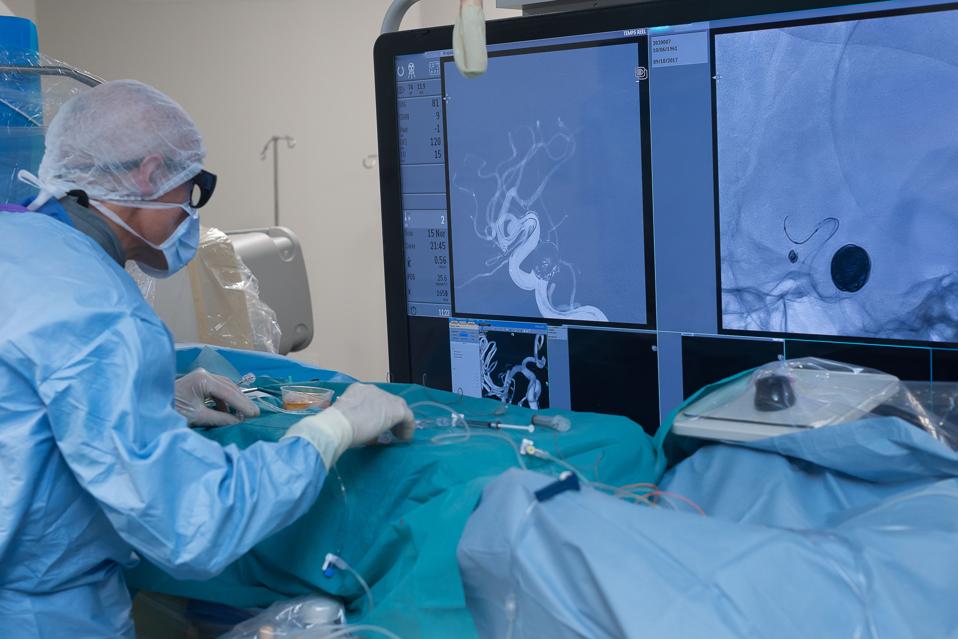 |
Filters are placed in the inferior vena cava (IVC) by interventional radiologists Universal Images Group via Getty Images |
"Sticky blood" is thought to be a result of the virus triggering a massive boost of cytokines, a protein that signals to the liver that increased clotting is required to be produced. Which has led scientists to the expectation that blood thinners like Aspirin may assist in avoiding deadly clotting. Proven effective, Aspirin use would become the first over-the-counter drug known to have an important impact on one of COVID-19's deadly threats to those affected with serious consequences.
The committee was apprised of acetylsalicylic acid's potential success as a vital therapeutic tool, and Professor Horby's intention to have his team research its effects. Late last month a study was published by the University of Maryland, that COVID-19 patients taking a daily low-dose Aspirin in protection against cardiovascular disease were seen to have a significantly lower risk of complications and death than those not taking the drug -- that Aspirin users were less likely to be admitted to an intensive care unit or to be hooked up to a ventilator.
They were also more likely to survive the SARS-CoV-2 infection, in comparison with patients admitted who had not been on an Aspirin regimen. Despite these findings, they are considered to be merely observational, leaving it unknown whether giving Aspirin more widely would be beneficial, since the U.S. has not conducted any trials into Aspirin use as a therapeutic tool in COVID-19 to aid against the development of blood clots.
 |
| Credit: Shutterstock |
Labels: Aspirin, Blood Clotting, Blood Thinners, Embolism, Research, SARS-CoV-2

0 Comments:
Post a Comment
<< Home