When The Experts Get It Wrong
 |
| People wait in line to receive their COVID-19 vaccination at a clinic in the Fraser Health region of Surrey, B.C., on Wednesday. New early data suggests that Canada’s recommendation to delay the second dose of COVID-19 vaccines for up to four months may not be effective in some older, more vulnerable people. (Ben Nelms/CBC) |
"People who want their second shot are now in limbo. Some don't know when their next shot is coming, some [are] told four months. They don't know if they have sufficient protection or if they have to wait, or whether the second shot will work.""They signed a consent, which included a vaccine information sheet that says in several places, three weeks for the second Pfizer shot and 28 days for Moderna. [This could be a problem for governments resulting from the legal principle of] detrimental reliance [where one party can force another to abide by its obligations under a contract]."Toronto lawyer Ian Cooper"Pfizer could be held legally liable for a death caused by an extension of the time between vaccine doses. I doubt that would ever happen.""I think it highly likely that Pfizer has been given some 'undertaking' by the Canadian federal government that will hold them harmless. Pfizer would have been legally wise to require that to be in place."David McCarthy, founder, pharmaceutical industry consulting company"The dosage of the Pfizer vaccine for the second dose is in three weeks, perhaps at the most in four weeks, in order to be effective -- 95 percent, based on clinical studies.""The delay by our government to postpone the second dose by an extra three months, to July, is irresponsible and dangerous.""The prime minister should advance the administration of the second dose, from July to April or May."Leslie Dan, founder of Novopharm Pharmaceuticals
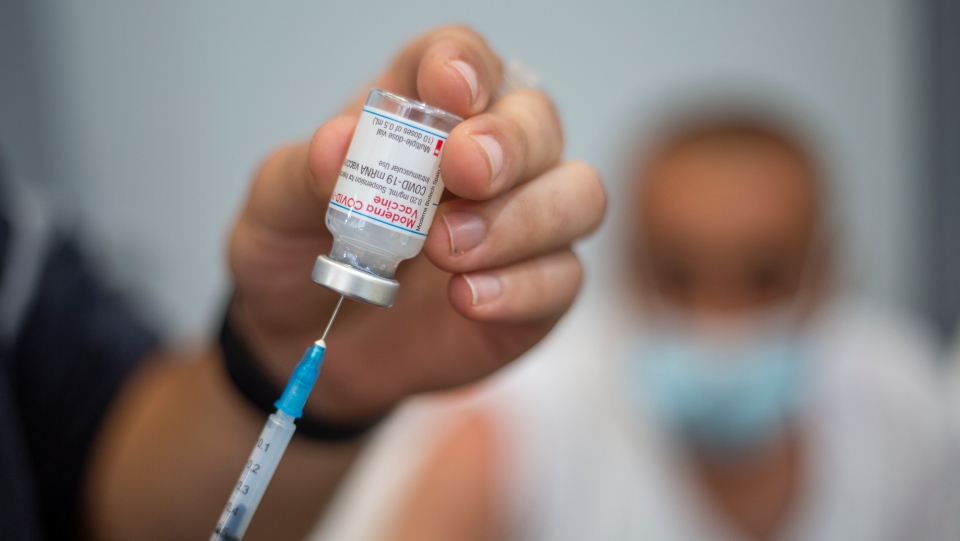 |
Canada became the sole country in the world earlier this month to mandate a gap of four months between doses of the Pfizer and Moderna vaccines. The country is in a position of severe vaccine shortage. Mostly resulting from the federal government's delay in ordering vaccines from trustworthy pharmaceutical sources. Prime Minister Justin Trudeau had directed his officials and federal health agencies to work in tandem with a Chinese pharmaceutical company, CanSino Pharmaceuticals which had a link to Canada since its founder was educated and worked for a Canadian pharmaceutical company before returning to China.
The National Research Council allowed CanSino unrestricted use of its biopharmaceutical platform on which to base its vaccine. The arranged contract, however, fell through when Beijing refused to permit the vaccine to be shipped to Canada where it was to undergo third trials and production. In the face of the reality of resulting late-order vaccine shortages from Western-oriented reliable sources, the National Advisory Committee on Immunization that had been appointed by the government, decided to stretch available resources by delaying second dosages in favour of vaccinating more people with a first dose.
This decision was undertaken on the theory that while the completed vaccine regimen of two doses reduces risk of infection by over 95 percent, data indicated the first shot on its own can reduce a patient's risk of contracting COVID-19 by a still-impressive 80 percent, ensuring a good level of protection from the virus despite failing to adhere to the 21-day recommended schedule. "Extending the interval between doses was shown to be a good strategy through modelling, even in scenarios considering a six-month interval and in theoretical scenarios where waning protection was considered", the Committee wrote.
 |
| Guido Armellin, 86, receives the COVID-19 vaccine during a clinic at a church in Toronto on March 17. A team was on-site administering the Pfizer-BioNTech vaccine to parishioners as part of a community outreach program to get seniors vaccinated at their place of worship. (Evan Mitsui/CBC) |
Data exists, however, indicating that a delayed second dose can present a disproportionately risky threat to the elderly, most vulnerable to COVID-19. Britain's Public Health Agency shows COVID-19 vaccines not to be as efficaciously potent in elderly populations. First shots for the elderly shown to provide 57 percent protection over the age of 80 in Britain, where 85 percent protection was achieved only when the second dose was administered.
Members appointed to Canada's National Advisory Committee on Immunization were found to be composed largely of pediatric specialists. "The overwhelming majority of deaths among COVID-19 victims have been elderly, and their interests and unique health and immunological considerations do not appear to be adequately represented on the NACI Working Group", wrote Toronto lawyer Ian Cooper, representing two retired elderly physicians threatening to take Canadian pandemic authorities to court over the decision to delay the second dose of Pfizer, the vaccine they also recommended for use in the elderly.
A notice of claim drafted by Ian Cooper representing the two retired elderly physicians, aged 79 and 83 who have expressed their opposition at having to wait a pharmaceutical-unauthorized protracted length of time for full vaccine coverage was forwarded to the Public Health Agency of Canada, the National Advisory Committee on Immunization and the federal and Ontario ministers of health. "The province broke the promise that was made both in writing and orally at the vaccination site", the draft notice of claim reads; conceivably prior to lodging a legal suit on behalf of the complainants insisting on being vaccinated in a "timely" fashion.
To further emphasize the inadequacy of the policy stretching out those unfortunately scarce vaccine resources, the head of the University of Ottawa Heart Institute has made an urgent appeal for all patients in treatment at the Institute and all patient-facing health workers to receive second doses of vaccine without further delay. Although those involved have received their first dose, in the interval between the first and the second both patients and health-care workers have contracted the novel coronavirus. "I am very worried for the staff" said Dr.Thierry Mesana, president and chief executive of the Heart Institute, at the very time that more transmissible variants are on the rise.

"Health Canada has the statutory mandate to authorize drugs for sale in Canada, and only it can authorize changes to the time interval between injections, and then only after the sponsor's submission of new data to support such a change.""Direct questions need to be asked to Dr. Supriya Sharma, chief medical adviser to the deputy minister and senior medical adviser, health products and food branch -- and specific answers are required.""Our politicians have played very loose with defined legal requirements, lied to Canadians and jeopardized our lives."Conservative Member of Parliament Michelle Rempel-Garner"I’m not aware of data showing that there is efficacy beyond two months of the first dose.""In the past few weeks, we’ve seen different studies come out showing that the response to the first dose of the vaccine in the people who are elderly, in the people who are immuno-compromised is actually not that good and it wanes quite rapidly.""[Both Health Canada and the NACI will also have access to the updated findings, meaning a new recommendation could be on the way]. I’m sure they’re following this and they may well be looking at perhaps modulating the recommendation as we go.""As data emerges about what it takes to protect [seniors and immune-compromised people], we need to be reviewing what we’re doing.""[As long as there aren’t issues with the vaccine supply, reverting to the original recommendations from the pharmaceutical companies] would be the ideal approach.""The very people we want to protect the most require that we give them the second dose using the shorter interval, originally as done by the manufacturer in the clinical trials. The one-size-fits-all approach really needs to be modulated in terms of who we need to be protecting."Canada’s chief science adviser Dr. Mona Nemer
Labels: 2nd Dose Delay, Canada, Controversy, Global Pandemic, National Advisory Committee on Immunization, Vaccine Supplies, Vulnerable Elderly

0 Comments:
Post a Comment
<< Home