COVID Spit-Testing
"When everything fell apart in the middle of March, I wondered if those kits might work for COVID-19.""In our study, the swab test detected more cases than the spit test. However, we feel that because the spit test is so much easier, it may still have a place in COVID-19 testing in certain situations. Further research is crucial in this rapidly changing field.""Saliva testing brings another tool into the tool box to add to the testing options. I am a cancer researcher who happened to know about a spit test. My other areas of interest have slowed down while everybody's focus is on this [the novel coronavirus].""I find it very interesting to be part of potentially some of the knowledge that helps in this pandemic. I think that is super rewarding."Dr.Stephanie Johnson-Obaseki, head and neck cancer surgeon, The Ottawa Hospital
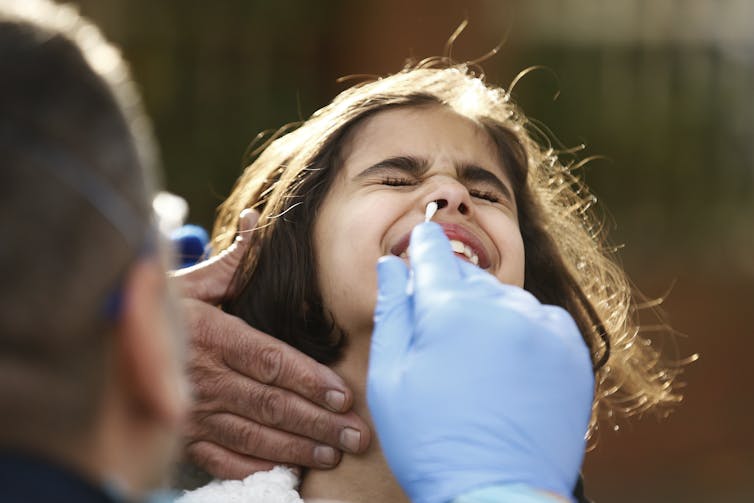
| Provincial health workers perform a nasal swab to test for COVID-19 on Raymond Robins of the remote First Nation community of Gull Bay, Ont., on April 21. Proponents of saliva-based testing say it is easier to administer — and less uncomfortable. (David Jackson/Reuters) |
"I think it comes down to public health policy and our approach to testing. If we believe the right approach is to test more and reach more individuals, not only those who are sick but also asymptomatic, this is a way to reach those individuals."Rafal Iwasiow, VP, Innovation and Technology, DNA Genotek"Faced with an ongoing lack of protective equipment and testing supplies, medical professionals have been seeking alternatives to accurately diagnose cases of COVID-19, a pandemic that has caused more than 11 million cases and more than 530,000 deaths worldwide. Supplies of nasopharyngeal swabs were some of the first testing materials to run low in mid-March, prompting a pivot to nasal swabs. More recently, saliva-based testing has come forward as an attractive, low-cost alternative. The first spit tests are already being sold to consumers, with more poised to apply for emergency use authorization from the US Food and Drug Administration soon. While saliva can be a crude sample for diagnosing disease using traditional PCR, it pairs well with a cheap PCR alternative known as loop-mediated isothermal amplification (LAMP), previously used to detect outbreaks of Zika and Ebola in resource-poor countries. Propelled by a global pandemic, researchers in the US and the UK are now modifying LAMP and assessing its utility as a diagnostic tool for COVID-19." Amanda Heidt, TheScientist
 |
ABOVE:
A new test from Columbia University analyzes saliva for genetic traces
of SARS-CoV-2 using LAMP. Samples that contain viral RNA change color
from red to yellow. |
Normally, Dr.Johnson-Obaseki researches human papillomavirus in head and neck tumours and had gained experience working on HPV testing with the use of kits designed by DNA Genotek. Then the pandemic arrived and research other than that linked to SARS-CoV-2 receded, and her thoughts turned to what she knew about those saliva-testing kits. Not only did she wonder if they would be useful in testing for COVID, but she discovered they did work, then made use of them to study just how feasible spit testing would be for COVID-detection.
While slightly less sensitive than nasal swabs, Dr.Johnson-Obaseki and other researchers published a paper describing use of the tests and their usefulness, with an emphasis on remote communities and populations as an example in long-term care residences and for use with students. The nasal swabs are extremely uncomfortable, albeit more accurate, while the spit test is simple and direct and quick. Their study, published in the Annals of Internal Medicine took data from 2,000 people who presented for testing at the COVID-19 assessment centre at The Ottawa Hospital.
 |
After undergoing the standard nasal test, the participants agreed to signing on to a self-administered saliva test. Of that total of participants, 70 tested positive for COVID-19, 34 were positive on both the nasal swab and the spit test, 22 were positive only on the swab test and 14 were positive on the saliva test alone. Convincing enough for the researchers to agree that the spit tests were functionally dependable on their own.
 |
Spit tests are universally put to use in reflection of their ease of use, and can be self-administered, as a bonus. Avoiding the use of a nasal swab which can be an aversive experience for many, in particular people who must undergo a series of such tests, represents another issue in favour of spit tests. At the present time, such tests are available in Canada only for experimental use. Health Canada indicates it now is considering approving these test kits for screening only, not for diagnostic purposes.
Health Canada is not yet prepared to authorize saliva sample use with any authorized testing device, stateing it is "actively working with companies who have filed their application for saliva-based testing and will prioritize the review of applications for test kits that use saliva samples in order to enable new testing options for Canadians." As departmental research chair in quality improvement at University of Ottawa, Dr.Johnson-Obaseki feels the ease of the spit test in comparison with nasal swab testing is a large point in its favour.
In August, the U.S. government gave emergency authorization to a saliva COVID-19 test that Yale University in conjunction with the NBA and its players union developed. The Yale test could miss patients with low levels of the virus, researchers say, but regular testing should offset that issue. Test kits, including those produced by DNA Genotek have been given emergency authorization in the U.S.
 |
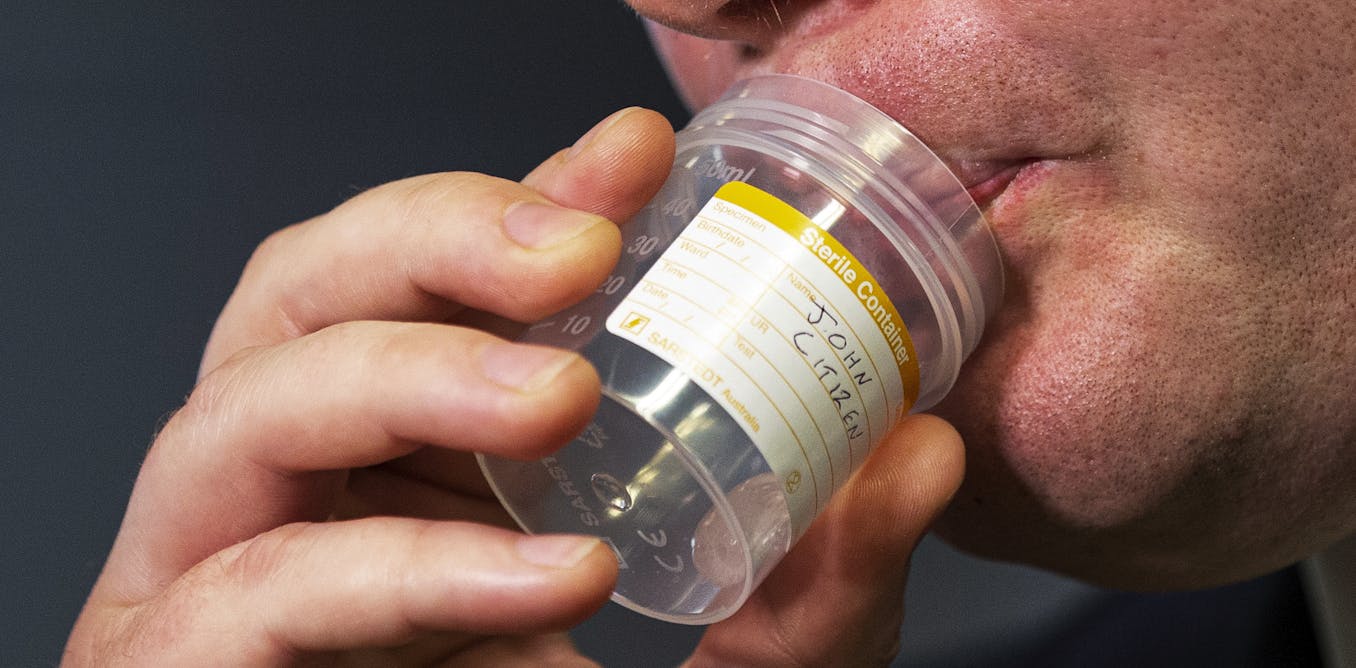
| A student in California provides saliva for an experimental COVID-19 coronavirus test for asymptomatic people. Some American universities have also started offering saliva-based options to test students. (Irene Yi/UC Berkeley via AP) |
Labels: Canada, COVID-19, Spit Testing, The Ottawa Hospital

0 Comments:
Post a Comment
<< Home