Time-Anxiety in COVID-Vaccine Release, Efficacy, Safety
"Certainly there can be a lot of damage done between now and when vaccine programs are rolled up.""So, we really hope that the provinces and municipal, political and public health leaders take steps to protect people. And I hope people take steps to protect themselves and their communities."Isaac Bogoch, infectious disease specialist, Temerty Faculty of Medicine, University of Toronto
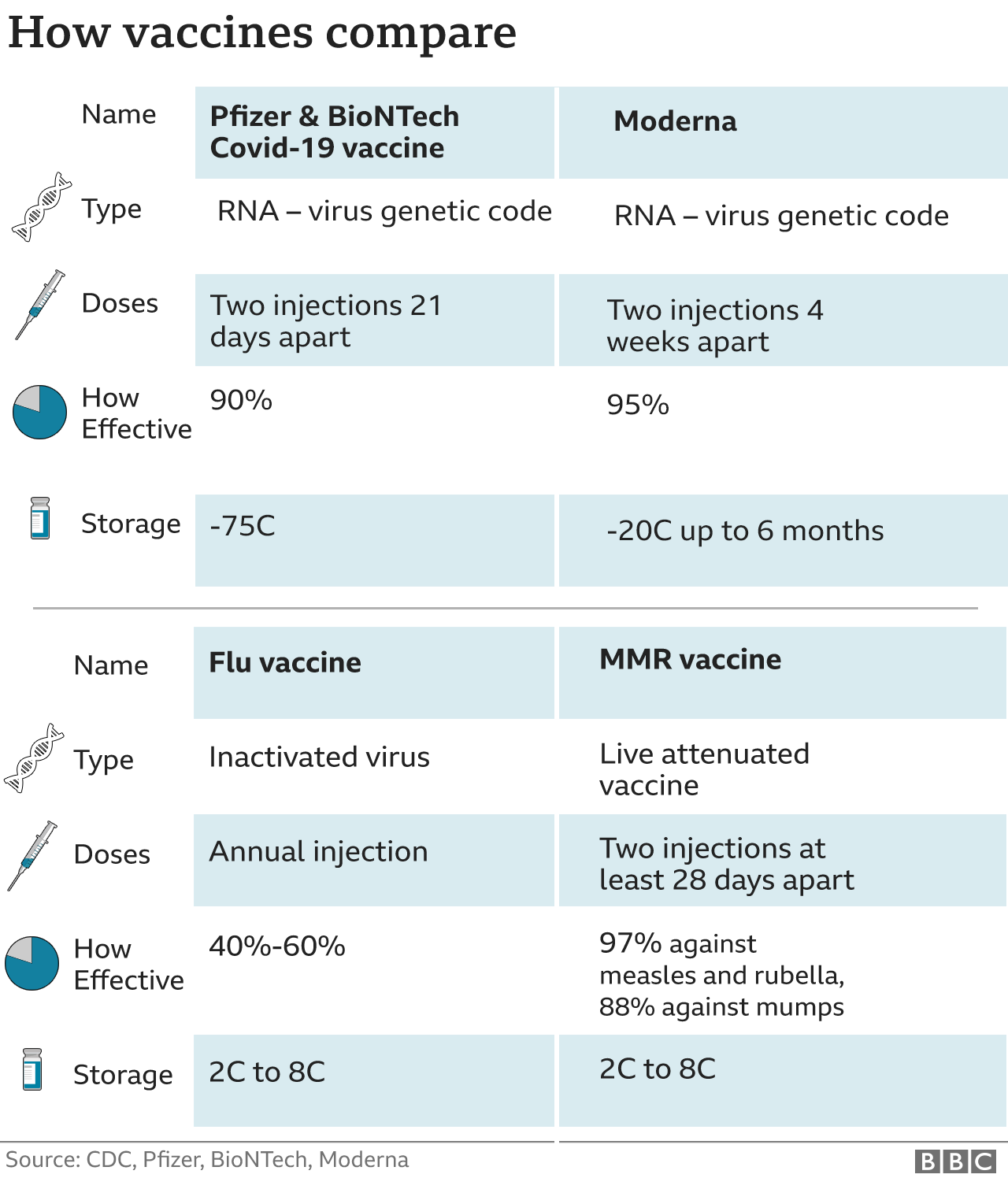
"[The preliminary results for both Pfizer and Moderna vaccines are] incredibly exciting. Both have reported over 90 per cent efficacy, which is really extraordinary and really meaningful. But we do have to remember that these are both based on short-term results after having been immunized.… Would that efficacy hold up over a period of weeks, months or even years?" "We think we see the light at the end of the tunnel here. We think that there's something that can be added to the toolbox that can really make a difference and change the shape of this curve and the experience that we've all been living through." "Those are the things that will be huge game changers [whether these vaccines alter the transmission of the virus, including the length of infection, the level of virus in the body and how likely an infected individual is to transmit to someone else]. If the vaccine can alter any of those attributes of the virus." Kate O'Brien, director of the immunization department, World Health Organization (WHO)

"... It’s not a ‘recipe’ that you can make the material out of. It took us years to learn how to make the vaccine and months to train the Lonza people [Moderna’s European manufacturing plant, which is owned by Switzerland-based Lonza] on how to produce it. We’re sending our best people to teach the process and like any young technology, it takes time; it’s a difficult project that involves a lot of transfer of patents. A lot depends on the raw materials and the production efficiency and this is the first time the vaccine is being produced.""All of that [trust in the trial results and decision to release results] is managed by an independent committee that examines the data and decides when to approve publication. The figures were clear even when there were 53 Covid-19 cases in the experiment, but we waited until we had 95.""The [test] subjects are living completely ordinary lives, some even work in the healthcare sector. They are all exposed to the virus. In any case, the results are clear and indicate the vaccine is 94.5% effective.""At this stage, it is too early to tell [whether people who have been vaccinated can no longer transmit the virus], these are figures that we’ll have to examine when we test the blood of vaccinated people for antibodies."I don’t think that’s reasonable [conjecture that the vaccine might last only 3 months], there are very high levels of antibodies in the blood that we saw in the clinical trials, and that provides a sense of security and optimism. Of course, we will have to monitor the test subjects, but the high effectiveness rates offer a light at the end of the tunnel."Dr. Tal Zaks, chief medical officer, Moderna
| Staff are seen Sept. 29 setting up a production line in Visp, Switzerland, where the Moderna mRNA coronavirus disease (COVID-19) vaccine will be produced. (Denis Balibouse/Reuters) |
So the pot of gold at the end of the rainbow might be in sight, once the storm of COVID-19 assaults meets what will be its nemesis, arming vulnerable human beings against the onslaught of SARS-CoV-2 virus causing COVID. The actual proof of its long-lasting effectiveness safely encountered will have to stand the test of time. And in the meanwhile, a restive and fearful, angry and resentful, anguished and bewildered population must bide its time while continuing to follow medical instructions to maintain physical distance, wear face masks and observe carefully protective hygiene protocols.
The normal course of developing effective, safe vaccines has been reduced dramatically in the face of this fast-moving, volatile and changeable virus that hasn't obeyed the hitherto-observed behaviours of predecessor viruses, leaving the scientific-medical community bemused and challenged to develop protocols of protective behaviours, asking entire populations to undergo dramatic modifications of what has always been considered 'normal' life. Long engrained habits have had to be abandoned. Social contacts of immense value brought to an abrupt halt.
But the good news is that following Pfizer's announcement that its vaccine has proven hugely successful and the pharmaceutical company is prepared to ask for emergency title to begin production and distribution, Moderna too has released similar triumphant news. And this at a time when the world is overdue for good news, while the second wave of COVID infection has proven to be far excessive of the original in its viciously predatory infectiousness.
 |
| Moderna scientists used an innovative technique for developing the vaccine so quickly Moderna |
Preliminary data of Moderna's Third-Stage trials give its mRNA-1273 vaccine a 94.5 percent effective rating, outdistancing even the unheard-of 90 percent effectiveness of Pfizer's vaccine. With an added advantage that Moderna's vaccine requires only typical refrigeration, and not the complicated -70C refrigeration of Pfizer's vaccine, making it easier to safely distribute and store. Both pharmaceutical companies' vaccines are pending additional safety data and regulatory review leading to a sooner-than-later authorization in the United States for emergency use, perhaps by December.
Both companies, apart from awaiting the verdict of the U.S. Food and Drug Administration in advancing their vaccines' permission to proceed to production and distribution have also asked Health Canada to review their products with a mind to similarly manufacturing and distributing for Canadian use, at a critical junction where infection case rates have been soaring in Canada, yet nowhere near the astronomical heights of infection seen in the United States.
"We are going to have a vaccine that can stop COVID-19", Moderna's president Stephen Hoge said with confidence. Only five infections were seen to occur in volunteers who received the two-shot (28 days apart) mRNA-1273 vaccine, out of a total of 95 receiving the vaccine or an alternative placebo. The company is confident that it will be in possession of sufficient safety data for U.S. authorities within the week or more to enable them to file for emergency use in coming weeks.
No one should be surprised, interjects infectious disease specialist Isaac Bogoch, if things don't exactly run as smoothly as expectations lead people to anticipate, while questions are left dangling awaiting answers; how long the vaccine may last, and how effective the inoculation will be in a real-world setting.
 |
Labels: Biotech Innovations, COVID Vaccines, COVID-19, Moderna, Pfizer

0 Comments:
Post a Comment
<< Home