A Paradigm Shift in Cancer Care
"This really is a paradigm shift in cancer care.""These immune-based therapies have really revolutionized the field and have shown these long-term, durable remissions.""It's a procedure done with close medical care.""We want to be able to deliver it with the greatest medical benefit, but also with a favourable financial risk profile -- especially in the context of the Canadian system [of tax-based universal medicare)."Dr.Michael Kennah, hematologist, The Ottawa Hospital"For patients who have relapsed or who have not responded to treatment, it really provides a transformative option.""These patients were previously facing a dire prognosis."Antonella Rizza, chief executive, Lymphoma Canada
 |
| Dr. Natasha Kekre works on Cdn CAR-T cancer therapy. |
The Canadian Cancer Society represents roughly 10,400 Canadians diagnosed with non-Hodgkin lymphoma yearly.This is a cancer that begins in the lymphatic system, the body's network for fighting disease and infection. White blood cells called lymphocytes develop tumours for which conventional treatment that includes radiation, chemotherapy and other therapeutic drugs are brought into action, and there are patients who also may undergo stem cell transplants. Among sufferers of this type of cancer a minority see their cancer resist all of these therapeutic treatments.
For the first time, a new cutting-edge personalized form of immunotherapy -- named CAR T-cell therapy offers hope to these patients for whom only palliative care was left after their cancers failed to respond to conventional therapies -- offers hope. In the past, adult patients undergoing recurrent or treatment-resistant lymphoma were given an average of six months for survival. Yescarta was approved by Health Canada in 2019, given encouraging data from a clinical trial and two years of followup studies.
Marketed under the brand name Yescarta, the new therapy represents the second such chimeric antigen receptor (CAR) T-cell therapy approved for use in Ontario. Now, two therapies, Kymriah and Yescarta are available for treatment at The Ottawa Hospital for the first time, for these cancers unresponsive to conventional treatment. Both come at a steep cost of hundreds of thousands of dollars for one-time treatments, meant to be used for a very small number of relapsed blood cancer patients along with patients suffering particularly aggressive forms of non-Hodgkin lymphoma.
 |
| Hematologist Dr. Jill Fulcher confirms that Stefany Dupont is in remission over one year after her CAR-T cell therapy. Previously, Stefany was given a 10 to 20 percent chance of survival, pre-CAR-T cell treatment. |
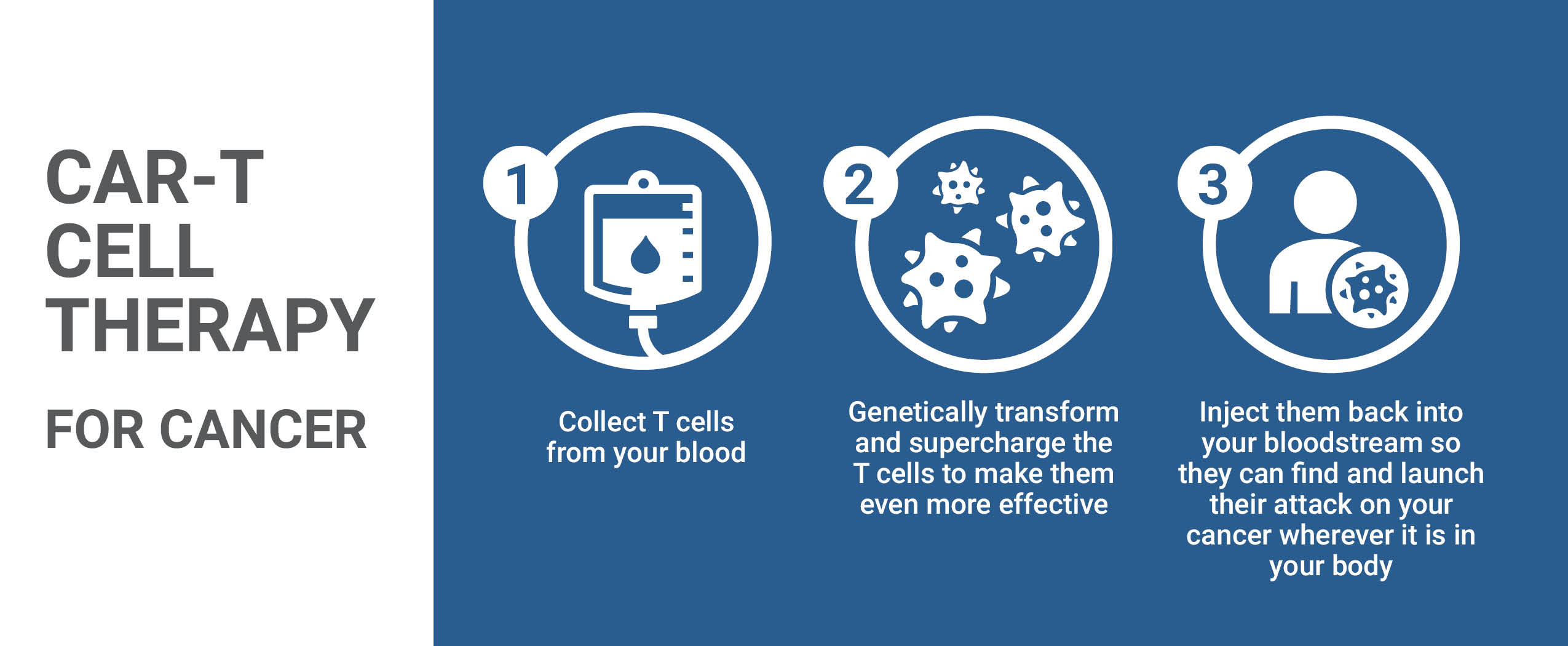
Through the study it was found that 74 percent of 101 adult patients who had been treated once with Yescarta responded, and 54 percent achieved complete remission. This high-tech form of personalized medicine using CAR T-cell therapy stimulates the power of the body's own immune system to be enlisted to fight cancer. The T-cells are separated from a patient's blood with the use of a highly specialized machine, after which the cells are forwarded to a laboratory in the U.S. to be genetically re-engineered.
In the process an inactive virus is manipulated to insert genes into the cells to create artificial receptors -- chimeric antigen receptors (CARS) -- on the cells which equip the T-cells with the capacity to recognize and then attack a specific protein on the patient's tumour cells. The altered T-cells are laboratory-multiplied to enable hundreds of millions to be infused back into the patient. Nothing, however, comes without risks; the therapy can trigger potentially deadly side-effects.
A process called cytokine release syndrome (CRS) can emerge as a reactive side-effect, where dangerous levels of cytokines -- a chemical messenger -- are released into the bloodstream. It is this possibility of the emergence of such a side-effect that mandates the treatment to be given as hospital in-patients for monitoring purposes. Staff at the hospital were given thorough extensive site training before they could be certified to deliver the two CAR T-cell therapies.
Around 20 out of 100 patients annually who are diagnosed with diffuse large B-cell lymphomas at the hospital resist front-line and secondary therapies, some of whom are now eligible for Yescarta treatment. In an effort to determine which lymphoma patients may be the best candidates for CAR T-cell therapy and the optimal time to treat them, hundreds of studies are taking place globally, advises Dr. Kennah.
The personalized therapies come at an immense cost, from a half-million per treatment down to three-quarters of a million per patient. Kymriah, a CAR T-cell therapy for acute lymphoblastic leukemia in children and young adults who fail to respond to conventional therapies is available now in four certified Ontario hospitals. Kymriah can be used as well to treat an aggressive form of non-Hodgkin lymphoma. At the present time Ontario, Alberta and Quebec finance the Kymriah cost while Ontario is the first to fund Yescarta as well.
Labels: CAR T-cell Therapy, Conventional Therapies, Non-Hodgkin Lymphoma, The Ottawa Hospital

0 Comments:
Post a Comment
<< Home