What's The Rush? Lives Depend On It
"For the initial stages of vaccination, I can confirm that we are asking for vaccines to be administered only at the first vaccination points."Christina Antoniou, spokeswoman, Pfizer"Whatever storage and transportation conditions are used for the vaccine, studies have to have been conducted in advance to demonstrate that the quality of the vaccine is maintained under those conditions.""The challenge is that you have a limited amount of time to run those studies, which are relatively complicated.""The messenger RNA is very fragile after it’s been administered, it’s broken down very quickly in the body, and it also can’t get into our cells where it needs to be to work. So we provide a delivery technology [to Pfizer] that protects the messenger RNA after it’s administered and carries it into the cells and then releases it in the cells so that it can work.""We don’t have the ability to do long stability studies to establish a shelf life for the vaccine at a more convenient temperature."Thomas Madden, chief executive, BioNTech partner Acuitas Therapeutics, Vancouver"So if you do have an urgent situation such as this, you can have this rapid approval process that’s not technically a full approval process.""[It] really says, you know what, we’re still going to do the full look, we’re still going to do the full approach [the difference between the normal vaccine approval process and the fast-tracked process] to ensure that this is a safe and effective product."Infectious diseases specialist Dr. Isaac Bogoch, University of Toronto
| Approved by Health Canada, the initial batch of the Pfizer/BioNTech vaccine would be enough for nearly 125,000 Canadians. (Carlo Allegri/Reuters) |
Two days ago, Health Canada, under obvious pressure took the step of clearing Pfizer and BioNTech's COVID-19 vaccine for public use; this extraordinary step even before the U.S.'s regulatory body made their decision for the U.S./German-produced vaccine. It is highly unusual for Health Canada to take such an accelerated approach to approving any new drug; it usually drags on through the approval process long after the U.S. Food and Drug Administration has arrived at its approval status.
Canada's experience with COVID-19 has been that the vast majority of seriously complicated COVID cases and 80% of COVID-related deaths have been within the elderly demographic, residents of retirement homes and long-term care facilities. An advisory panel to the government recommended that long-term care home residents and health-care workers be prioritized for early vaccinations. And that was to be the plan going forward.
With the imminent arrival of Canada's first modest-in-numbers batch of the Pfizer vaccine, the realization of the simple fact that to remain stable, the vaccine's fragility which requires extreme refrigeration from -70 to -80C, would make it not only a logistical nightmare to inoculate the elderly in these institutions, but aboriginals -- also declared priorities for vaccination -- living in remote, isolated communities would have to await a different type of vaccine; one produced for example by Moderna which can be stored at a moderate -20C.

Despite the urgency to vaccinate the elderly, the health-and-immunity-compromised, the health-frail indigenous populations, the vaccine originally designated in principle for them, will only be available for those able to take themselves to central designated vaccine-administrating stations to receive inoculations of the first deliveries expected to arrive in the next few days. Advice of a restrictive nature from the drugmaker has altered the best laid plans of health and government agencies.
Pfizer advising that early doses of the vaccine be administered solely at the sites where they have been delivered originally in large batches. This, given an absence of complete data on the vaccine's transportability safety. Nor, in he absence of definitive data, will it be possible to break up batches to send smaller quantities of the vaccine to other locations. Depriving many long-term care residents of the first vaccination batches in Canada, in the very midst of an infection surge on the cusp of overwhelming hospitals.
This delay equates to the risk of increasing COVID deaths despite the vows taken by health and government authorities after the appalling death toll of susceptible seniors, that heaven and earth would be moved to protect them in future. There has been no secret of the need to store the vaccine at ultra-cold temperatures, despite which the newly-revealed restriction in transporting the vaccine further along the chain from its original destination has struck a blow to those plans. Even physical movement like shaking during vaccine handling has been cautioned against.
The Canadian government has identified 14 initial points of vaccination and only there is Pfizer prepared to deliver its batches destined for Canadians. No reason was given by the company for its imposed restriction. The challenge for administrators to ship vaccines from their original reception point to facilities elsewhere has been answered; it cannot, as yet be done. Dr.Madden, chief executive of Acuitas Therapeutics points to a lack of data due to the rapid rollout, not necessarily the actual durability of the vaccine. Simply put: not enough is known for complete confidence in the vaccine's stability.
Dr.Madden, an expert in lipid nanoparticles, a key ingredient in the vaccine, made note of the fact that BioNTech offered less restrictive guidance in a presentation recently given during which it was stated that defrosted vials could be transported for up to six hours.
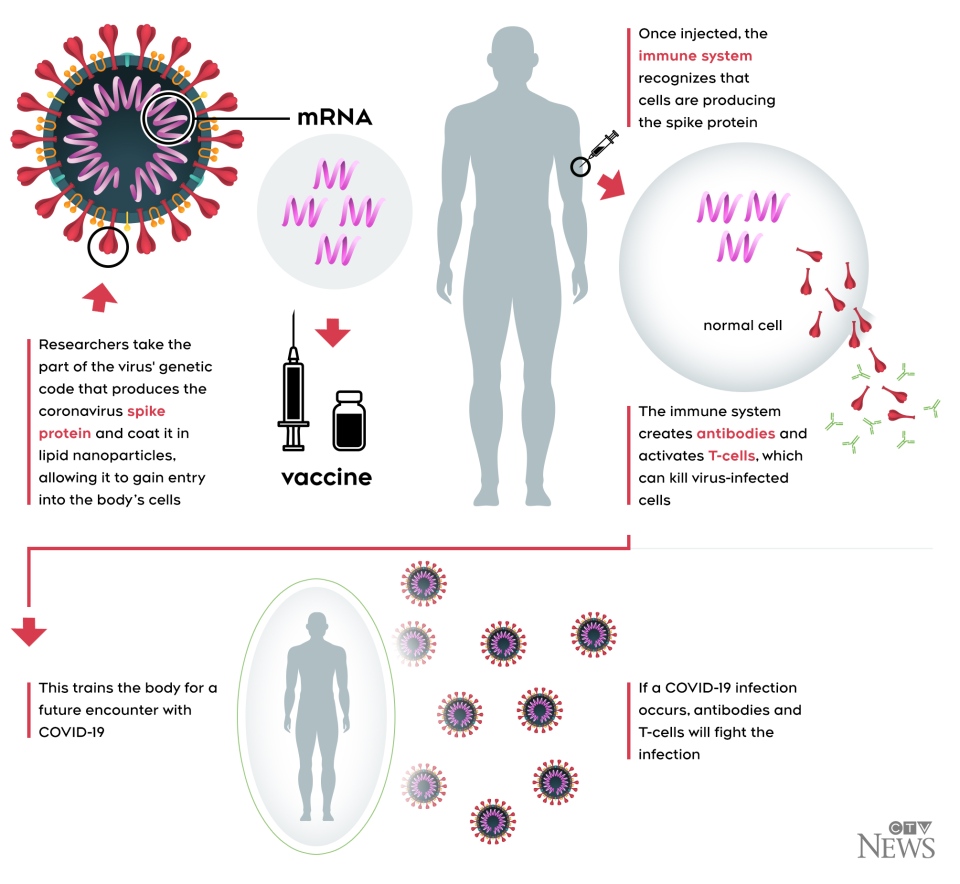
"But for now we’re going to do — I
wouldn’t say a cursory look, but it’s not a complete look."
Labels: Canada, COVID-19, Inoculation, Long-Term Care Facilities, Pfizer, Refrigeration, Vaccine Stability

0 Comments:
Post a Comment
<< Home