Achieved: The Holy Grail of Malaria Vaccine
"There are about 13 million children born each year in various areas of Africa that could really benefit from the malaria vaccine.""There are three doses per schedule and a booster dose so 200 million [the number the pharmaceutical manufacturer in India has agreed to produce yearly] is a very good number.""That's a real technical challenge. The vast majority of vaccines haven't worked because it's very difficult.""[However the trial results meant the vaccine was] very deployable [and] has the potential to have a major public health impact."Adrian Hill, director, Jenner Institute Oxford University"[The results were] very exciting [and showed] unprecedented efficacy levels"."We look forward to the upcoming 'phase III' trial to demonstrate large-scale safety and efficacy data for a vaccine that is greatly needed in this region."Halidou Tinto, professor in parasitology, principal trial investigator, Clinical Research Unit of Nanoro, Burkina Faso
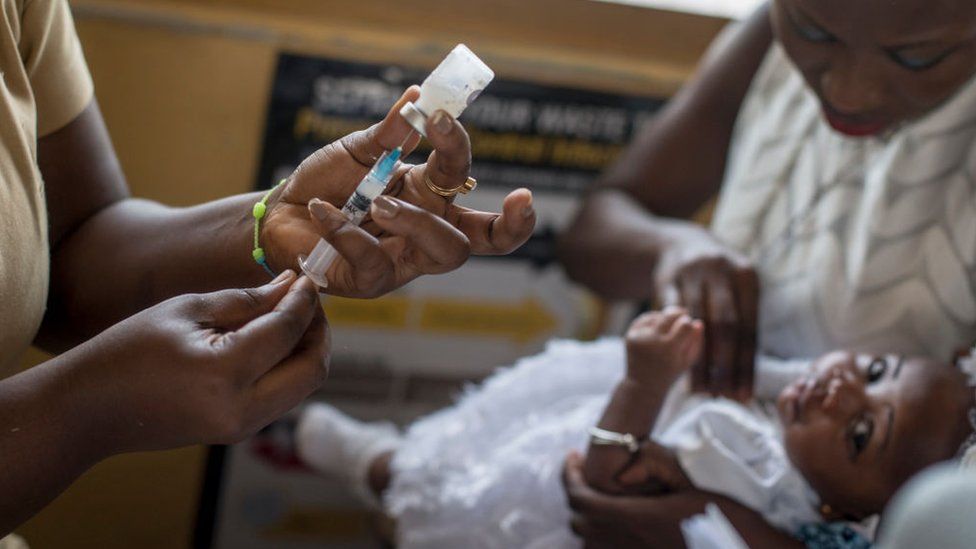 |
| Vaccines against malaria have been rolled out before, such as this one in Ghana Getty Images |
Malaria is a mosquito-borne disease that plagues the hot and humid areas of the world from Asia to Africa, the cause of sub-Sahara Africa losing approximately 400,000 people annually. Now, preliminary results of a phase two trial undertaken with 450 children in Burkina Faso indicates that the vaccine produced by scientists at Oxford University's Jenner Institute is 77 percent effective against malaria. The vaccine, called R21, is the first of its kind to rate such a high efficacy rate.
An agreement with the Serum Institute of India, one of the world's largest and most productive vaccine manufacturers, assures that they are committed to producing 200 million doses annually as soon as the vaccine has gained official approval. When that happens, the researchers anticipate that the vaccine doses will be available widely, and at low cost, to solve one of the world's most vexing environmental health risks through a vector that has always plagued humanity.
The existing anti-malaria vaccine, of promise, the RTS.S vaccine produced by GlaxoSmithKline, its efficacy rate came out to 50 percent in late-stage trials, the barest minimum to declare it a success. While this vaccine has completed phase three clinical trials it has not yet been fully licensed as a result of safety concerns. It is, however, being administered at the present time in Kenya, Malawi and Ghana, representing part of a World Health Organization-led pilot project.
Having a second vaccine in circulation would represent an immense assist to efforts geared toward eliminating malaria. Other preventive measures which are moderately effective include bed nets, indoor spraying, and preventive drugs, their use hampered by insufficient funding to ensure these methods are widely available where they are badly needed.
Children aged five to 17 months were given the R21 vaccine in three doses a month apart, with a booster dose a year later, in this latest trial. Three phase-three trials with 4,800 subject-participants for the vaccine are on the cusp of initiation in Africa. Challenge trials where volunteers in the U.K. were given the vaccine, then subjected to bites by malaria-carrying mosquitoes have earlier been completed.
Successful Malaria vaccines have been the goal of researchers for the past century, a goal that has been difficult to achieve. Partially as a result of the disease being seasonal, where 90 percent of deaths happen three to four months following the rainy season, emphasizing the vital importance of inoculating with vaccines at the precise right opportunity.

Labels: Africa, Anti-Malarial Drug, Children, Deaths, Malaria, Oxford University

0 Comments:
Post a Comment
<< Home