Will The Spirit Molecule Aid Recovery from Ischemic Stroke?
"The idea is can we stimulate neurogenesis without being psychedelic? And I think that's a really clever idea. No one knows. But if it works, it's very exciting.""How you keep it [N-Dimethyltryptamine] a sub-psychedelic dose is going to be challenging, and we've got some ideas about that, but also if people are conscious, they can talk to you. If they start to say 'I don't like the side effects I'm seeing, I'm thinking funny thoughts', you can just turn it off and literally within a minute the effect dissipates.""The barriers are still immense [to testing psychedelic drugs]. But we're hoping that the more research is done and the more clinical utility is demonstrated that the barriers begin to come down. At present they're enormous and they add vast costs but also time. Getting import-export licenses for these drugs is a pain in the butt. It can take months to get permission to move the source compound across to another country to do the testing.""Before I was sacked, no scientists in Britain would actually tell the truth about drugs, they didn't ever want to confront the fact that the biggest killer in Britain is alcohol. And after I was sacked everyone said, 'Of course it is. It's obvious. So why do we care about these psychedelics that aren't very harmful at all?' So the public completely swung around to us.""The fact that Oregon is now legalizing mushrooms, against federal law, against the UN Convention, tells us we won the argument in the public. But we've still got to win it with the politicians."Dr.David Nutt, psychiatrist, neuropsychopharmacology professor, Imperial College London
 |
When Dr. Nutt was the top drug adviser to the U.K. government his condemnation of alcohol as the drug responsible for untold deaths, along with tobacco, as opposed to mild-to-moderate psychedelic drug use that was in contrast relatively harmless, landed him in hot water. His candid assessment ran counter to political views and he was fired from his position with the government of the U.K. ten years ago for his opinion expressed publicly that alcohol and tobacco were more harmful to individuals and society than LSD, ecstasy or cannabis.
While the learned professor, now 70, is still teaching and doing research at Imperial College and remains involved in trials with psychedelic medicines, he is also a consultant on a new trial that may bring hope to the treatment of ischemic stroke. Most strokes are ischemic, occurring when blood flow to the brain becomes obstructed. Options for treatment remain limited. A study published in the Annals of Neurology documented over a thousand drugs thought of as potential neuroprotective therapies tested from 1957 to 2003, none of which tested as viable options for effective treatment.
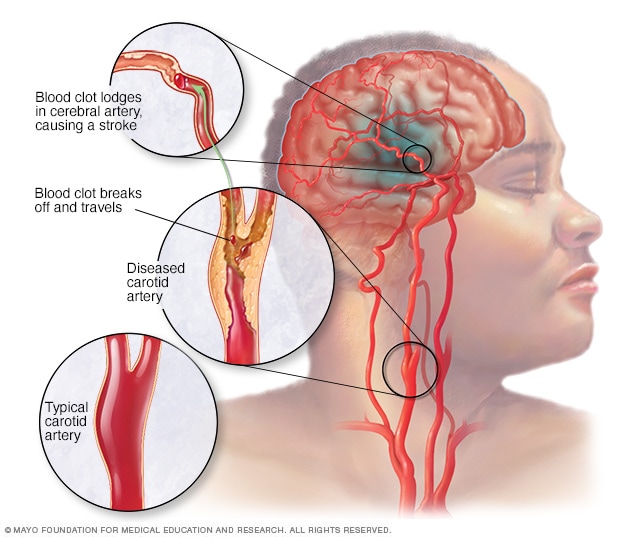 A clot-clearing drug, tissue plasminogen activator (tPA) was approved by Health Canada in 1999 for the treatment of ischemic strokes, but for the drug to be effective, it requires administration within several hours of the onset of stroke symptoms. Since tPA can also be deadly administered to someone who has experienced a hemorrhagic stroke, patients must wait for a CT scan before receiving treatment, a delay whose outcome can be devastating.
A clot-clearing drug, tissue plasminogen activator (tPA) was approved by Health Canada in 1999 for the treatment of ischemic strokes, but for the drug to be effective, it requires administration within several hours of the onset of stroke symptoms. Since tPA can also be deadly administered to someone who has experienced a hemorrhagic stroke, patients must wait for a CT scan before receiving treatment, a delay whose outcome can be devastating.Billions of dollars and decades have been spent while researchers searched for alternative drugs capable of preserving neurons and which in the best of all possible scenarios could be administered to patients without a scan being required. Algernon Pharmaceuticals based in Vancouver announced its plans to test the psychedelic compound recognized as belonging to the tryptamine family which includes substances like psilocybin, ketamine and LSD. DMT is familiarly known as "the spirit molecule". It occurs naturally in varieties of vegetation, some made use of in South America for religious ceremonies for centuries.
The pharmaceutical company anticipates that the drug will prove able to promote neurogenesis and neural plasticity to initiate new synaptic connections, and if it succeeds, stroke victims would be enabled toward faster recovery, with less ensuing damage. Continuous intravenous injection is visualized at a sub-psychedelic level so patients would not be exposed to hallucinations in its administration. Algernon submitted a proposal to the U.S. Food and Drug Administration respecting the design and scope of its preclinical and early phase stroke clinical programs.
Phase 1 of the trials purposes to establish dosages and the safety of the treatment, with the next phase to focus on recovery and rehabilitation. There is an existing foundation of pre-clinical studies demonstrating that DMT is capable of promoting neuroplasticity. The neuroprotective effect of DMT was demonstrated in a study published a year ago in the journal Experimental Neurology, with the use of an animal model. Once blood flow to the brain in rats was restricted, those treated with DMT suffered fewer lesions on the brain and recovered faster, with fewer severe effects.
 Algernon which harbours the expectation that its microdosing approach may serve to encourage a wider review and acceptance of the therapeutic potential of DMT, introducing the opportunity for further research, believes its approach could have the potential to lead to a Breakthrough Therapy Designation from the FDA, enabling priority review and fast-tracking of the clinical trial process. Dr.Nutt notes that these types of substances require rescheduling for real progress to occur. "If the schedules don't change, they're not going to be medicines, that's a fact."
Algernon which harbours the expectation that its microdosing approach may serve to encourage a wider review and acceptance of the therapeutic potential of DMT, introducing the opportunity for further research, believes its approach could have the potential to lead to a Breakthrough Therapy Designation from the FDA, enabling priority review and fast-tracking of the clinical trial process. Dr.Nutt notes that these types of substances require rescheduling for real progress to occur. "If the schedules don't change, they're not going to be medicines, that's a fact.""[I'm] hanging on to see sanity eventually prevail over the drug policy.""It is buzzing at present and I think that even if only half of the hope comes to fruition [it] is still going to be a hell of an event.""I hope I live long enough to see it all sorted."Dr.David Nutt, psychiatrist, neuropsychopharmacology professor, Imperial College London
Labels: Algernon Pharmaceuticals, Ischemic Stroke, Neurogenesis, Pharmaceuticals, Study, Therapy

0 Comments:
Post a Comment
<< Home