Steadily Emerging Biotechnology Options in Cancer Survival
 |
"For me, as a clinician, when my patients don't get access to innovative care, I hate that."
"Because I do bone marrow transplants, I am unfortunately in the business of telling patients that they're out of options and that they will succumb to their disease."
"Before CAR-T cells, I had that conversation many times. I still have that conversation, but it is less common now because at least I can say, 'We have the potential for CAR-T cells'."
"We realized we have the people, we have the infrastructure, we just have to put the parts together to build a CAR-T network."
"It's important to remember that CAR-T therapy is still very new and there can be serious side-effects. We need more research to learn about this therapy and make it work for even more people."
Dr.Natasha Kekre, associate professor, University of Ottawa
We depend on our immune systems to recognize biological invaders, and to begin the action for which nature designed them, to protect against those invaders, to mount natural internal defences for the purpose of countering the invaders and destroying them before they have the opportunity to destroy our bodies. In the case of cancer, the immune system fails to recognize cancer cells as invaders because those cells that have turned cancerous were part of us to begin with; defective cells that eventually turned cancerous.
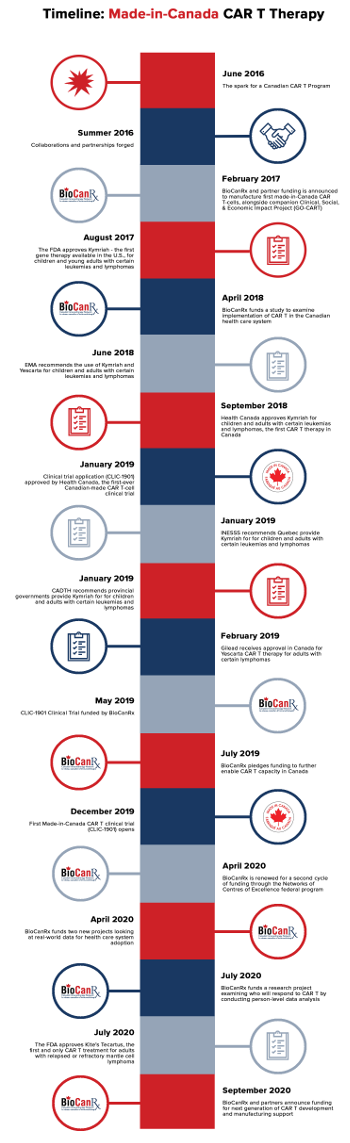
CAR-T, at the vanguard of the immunotherapy revolution represents an approach to fighting cancer by arming the body's immune system to recognize cancerous cells and to mount a defence against them. Cancerous cells have the capacity to evolve; through genetic alterations they develop the capacity to escape T cell detection. T cells are re-engineered through CAR-T therapy to find and attack cancer. The therapy is designed to make use of an inactive virus to insert genes into an individual's T-cells creating artificial receptors (chimeric antigen receptors; CARs), on the surface of tumour cells upon which chemical attacks are launched to destroy them.
Pioneering research in the field of CAR-T therapy was sited at Children's Hospital of Philadelphia where researchers working there become involved in a landmark study in 2014. The study saw publication in the New England Journal of Medicine in October of that year, reporting that 27 of 30 young patients with advanced acute lymphoblastic leukemia had gone into remission, responding well to the CAR-T therapy they were exposed to. Since that time research in CAR-T has accelerated to the point it now is one of the most studied and most promising of cancer research and development avenues.
In Canada two commercial CAR-T therapies have been approved for use; Kymrish and Yescarta, both meant to treat aggressive blood cancers that failed to respond to other treatments. A third CAR-T therapy, Tecartus was approved by the U.S. Food and Drug Administration last year for treatment of resistant mantle cell lymphoma. What all these treatments have in common aside from success as a last-effort treatment of huge potential is their costliness -- hundreds of thousands for one-time treatments.
Next step in new clinical trials, a focus on the technology's capability to engineer an immune system attack on solid tumours featuring advanced breast, prostate, pancreatic, lung and brain cancer. Hope is on the horizon, with over 150 clinical trials involving CAR-T therapy, recruiting in the United States. Few Canadians have been able to take advantage of such innovative clinical trials of the complicated-to-administer, expensive therapies.
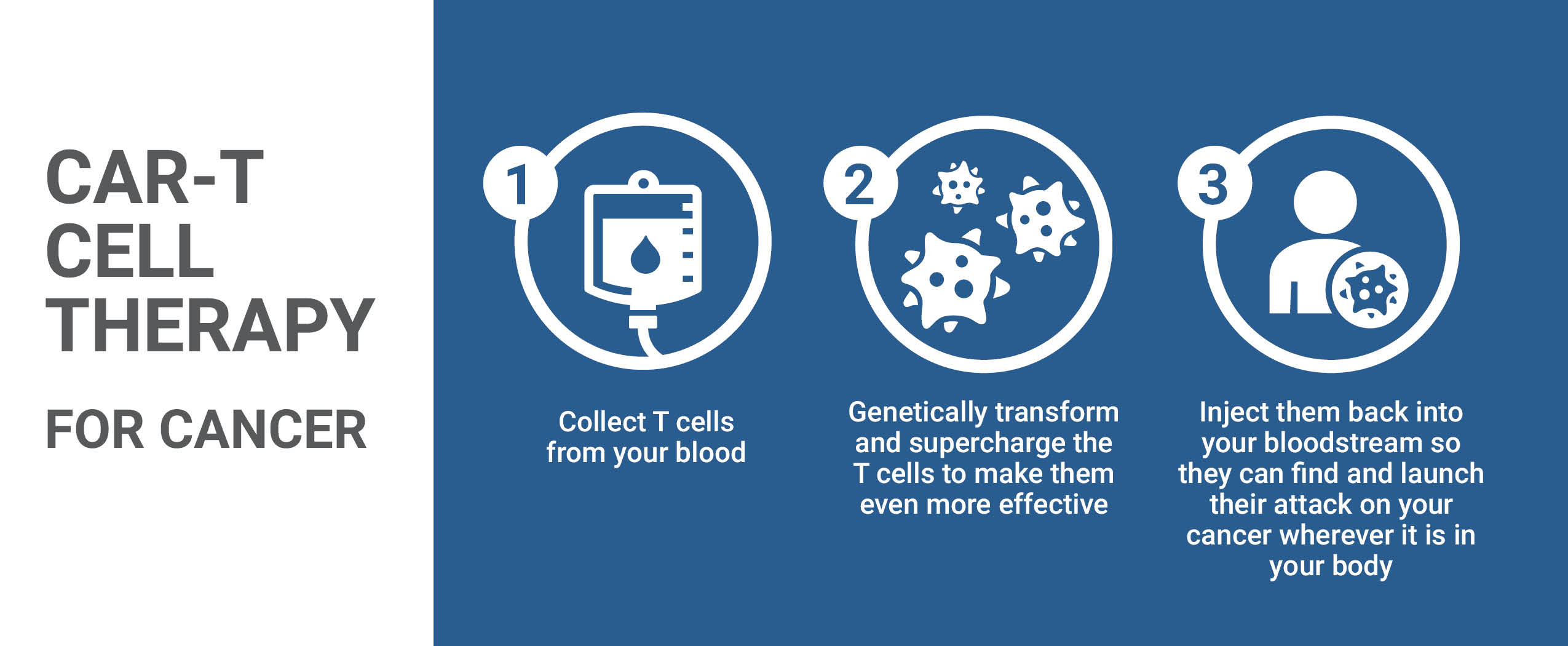
A situation that motivated The Ottawa Hospital scientist Dr.Natasha Kekre who saw no reason why Canada could not gather scientists in the field of medical biological therapeutic protocols across Canada to work together on this leading edge of molecular biology and medical technology, to create home-grown opportunities for Canadian patients. Where a patient's T cells -- white blood cells guarding against foreign invaders -- are genetically engineered to recognize and attack cancer cells.
There are now three Ottawa scientists dedicating their work to establishing a made-in-Canada CAR-T research network and a fledgling network is taking form with Dr. Kekre the lead investigator in the first clinical trial with Canadian-produced CAR-T cells. Back in 2016 at a immunotherapy conference Dr.Kekre invited other scientists from Ottawa, Victoria and Vancouver to a discussion to determine whether Canadian scientists could launch their own manufacture of CAR-T cells, conduct research and begin clinical trials.
The researchers all had established laboratories funded to address other projects. "We drafted out what we thought could happen -- how we could mobilize using the resources we had in Canada to make CAR-T cells here", explained Dr.John Bell, immunotherapy researcher at The Ottawa Hospital. Three laboratories collaborated to a) manufacture the required virus to deliver genetic material to a patient's T cells; b) produce the genetic material to be inserted into T cells; c) manufacture the modified CAR-T cells. "It was like a puzzle where all the pieces fit", stated Dr.Harold Atkins, stem cell transplant specialist, researcher at The Ottawa Hospital.
 |
Labels: Canada, Cancer Defence, Cancer Therapies, CAR-T Cell, Immune System, Immunotherapy, Research, The Ottawa Hospital

0 Comments:
Post a Comment
<< Home