Inoculating Children Against COVID : Closing the Gap
"We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the Delta variant and the substantial threat it poses to children."Albert Bouria, chairman, CEO, Pfizer Inc."This is a good-news story. This is great news, in the right direction, but there are still multiple extra steps before we see the vaccine in kids' arms.""It is going to take many weeks to review [Pfizer-BioNTech data], but probably not months.""This will require rigorous review. That might take some time to ensure it is done with the highest standards. But obviously we are all anxious."Dr.Anne PhamHuy, infectious-disease specialist, chair, Immunize Canada"I think it would be a huge deal for quelling some of the anxieties of parents. When it comes to keeping outbreaks out of schools, I think it would help enormously. And it gets us closer to having the herd-immunity conversation.""It is not clear whether herd immunity is possible. But, if it is, it can't be achieved without kids."Dr.Raywat Deonandan, epidemiologist, University of Ottawa
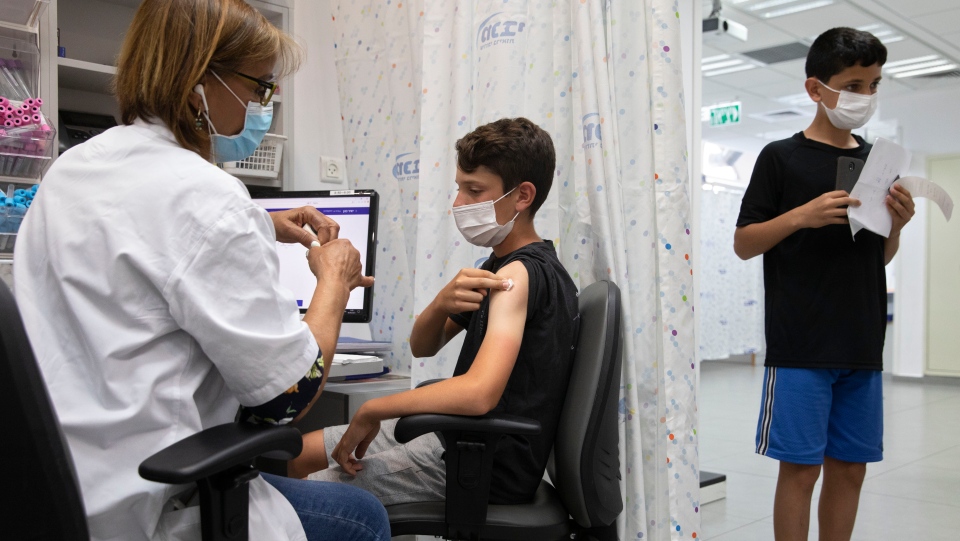 |
| An Israeli youth receives a Pfizer-BioNTech COVID-19 vaccine in the central Israeli city of Rishon LeZion, Sunday, June 6, 2021. Israel started vaccinating children from 12 to 15 on Sunday. (AP Photo/Sebastian Scheiner) |
Pfizer-BioNTech have announced that according to their clinical trials involving children of the ages between five and 11 their COVID-19 vaccine is safe, producing a robust immune response. Children in that age demographic were given a lower dose of vaccine -- one-third of the dosage given to older children and adults, and successfully produced antibodies to COVID, the signal measure of efficacy; with none suffering adverse effects.
The next step for the company is to apply for authorization from federal health agencies to enable the vaccine to be used for children in that age range in Canada, the United States, Europe and other places globally, wasting the least amount of time possible in a race against COVID.The announcement signalled the first indication that the vaccines should be available shortly for children. A reality of significant relief for both parents and the medical community.
That children under 12 years of age lacked an authorized vaccine is considered to represent a vacuum in an otherwise-well-covered area of safe and effective protection against the SARS-CoV-2 virus causing COVID-19, where the universal goal is to achieve herd immunity with the hopes of eventually moving away and out of the pandemic. As elsewhere, federal authorization must be achieved before young children can be inoculated.
 |
Only kids 12 and older are eligible — so far — to get vaccinated against COVID-19 in the U.S. But the shots could be available for younger children as soon as this fall, say researchers studying the vaccine in that age group. Chris O'Meara/AP |
Health Canada, speculates Dr.Pham-Huy, would likely receive Pfizer's application by late September or early October before a review of its data on safety and efficacy in children can move forward. Since the dosage for younger children is smaller than that for adolescents, it may take longer for the review to be completed.
The results from a Phase 2 and 3 trial indicated in participants from five to eleven years of age that the low-dose vaccine was "safe, well tolerated and showed robust neutralizing antibody responses", though the data has not yet been made public. As Dr.Deonandan explained the matter, Pfizer relies on antibody response in children in the trial as evidence of the vaccine's efficacy since there was insufficient data to calculate vaccine success.
In the tests conducted for adults the measure of success in vaccine efficacy was determined by how many test subjects among vaccinated and unvaccinated people become ill with the virus. An insufficient number of children contracted the virus in the Pfizer study to enable calculations to flow from data. Substituting that measurement of success with showing robust antibody levels in vaccinated children resulted in a "favourable safety profile".
 |
| The Pfizer/BioNTech
coronavirus vaccine is "safe, well tolerated" and produces a "robust"
antibody immune response in children aged five to 11, according to the
findings of a US trial. Getty Images |
Labels: Children, COVID-19, Pfizer-BioNTech, Research, Vaccines

0 Comments:
Post a Comment
<< Home