Hoping to Alleviate the Progression of Alzheimer's
"[The effect of the drug was] a modest one but I think it's clinically meaningful [since even a few months of delay in progression could give someone a little more time when they're functioning independently]."Dr. Ron Petersen, Alzheimer's expert, Mayo Clinic"When we met with patient advocacy groups, most of the time the first thing I said to them was, ‘Sorry'.""They wanted to know why it was taking so long, and were we not trying hard enough, or were we not intelligent enough?""We disappointed a lot of families."Ivan Cheung, chairman and CEO, Eisai Inc."If time is brain, and other [therapies] are taking eight to nine months to reach the optimal dose, patients are losing brain during that process.""With lecanemab, patients are starting from day one at the optimal therapeutic dose, and that is time-saving and brain-saving.”Dr. Sharon Cohen, medical director of the Toronto Memory Program
"Not all anti-amyloid drugs are the same. We are still learning what it means to mitigate amyloid dysregulation in terms of longer term benefit. But we now have an array of results with some encouraging signals that look like some of these drugs bully the disease process, and that is associated with some measurable clinical benefit for people with mild symptoms.""With my patients, I start with the analogy of a life raft that has a leak. Any medication will be a patch that slows down the leak, but doesn’t prevent it altogether.""For some of my patients, it’s helpful to think of drugs like these as a disease-slower-downer."Dr. Pierre Tariot, director, Banner Alzheimer’s Institute.
Scientists seem to be divided over whether a new drug to treat Alzheimer's is a breakthrough, or just another disappointment with little practical effect beyond giving the Alzheimer's sufferer a few extra weeks of something approaching normalcy -- at the very least functioning capability -- before the brain-destroying disease continues its ravaging effect leading to death. A new experimental drug slows the inevitable deterioration of the brain disease's effect, but it remains unclear what kind of difference in the final analysis it might make.
The drug Lecanemab, according to Japanese drugmaker Eisal and its partner, Biogen in the U.S., appeared to function well. To the present there has been one disappointment after another in the realm of discovery of a drug or therapeutic combination that might make a significant difference in alleviating the death sentence of Alzheimer's for its sufferers. The most common form of dementia, Alzheimer's diagnosis sends shivers and shudders down the spine of those afflicted, anticipating their inevitable decline..
Full results of the study with close to 1,800 people in early stages of Alzheimer's was released several days earlier by the two companies involved. The study, published in The New England Journal of Medicine, was also presented in San Francisco at an Alzheimer's meeting. Lecanemab was proven to delay patient deterioration by roughly five months, over the 18-month study's course.
Dr. Michael Irizarry stated that lecanemab recipients were 31 percent less likely to advance to the next stage of the disease during the study. "That translates to more time in earlier stages", he observed, when people are able to still function fairly well. Study participants received intravenous lecanemab or a saline infusion every two weeks of the study. With the use of an 18-point scale to measure cognition and functional ability, researchers tracked the subjects' progress.
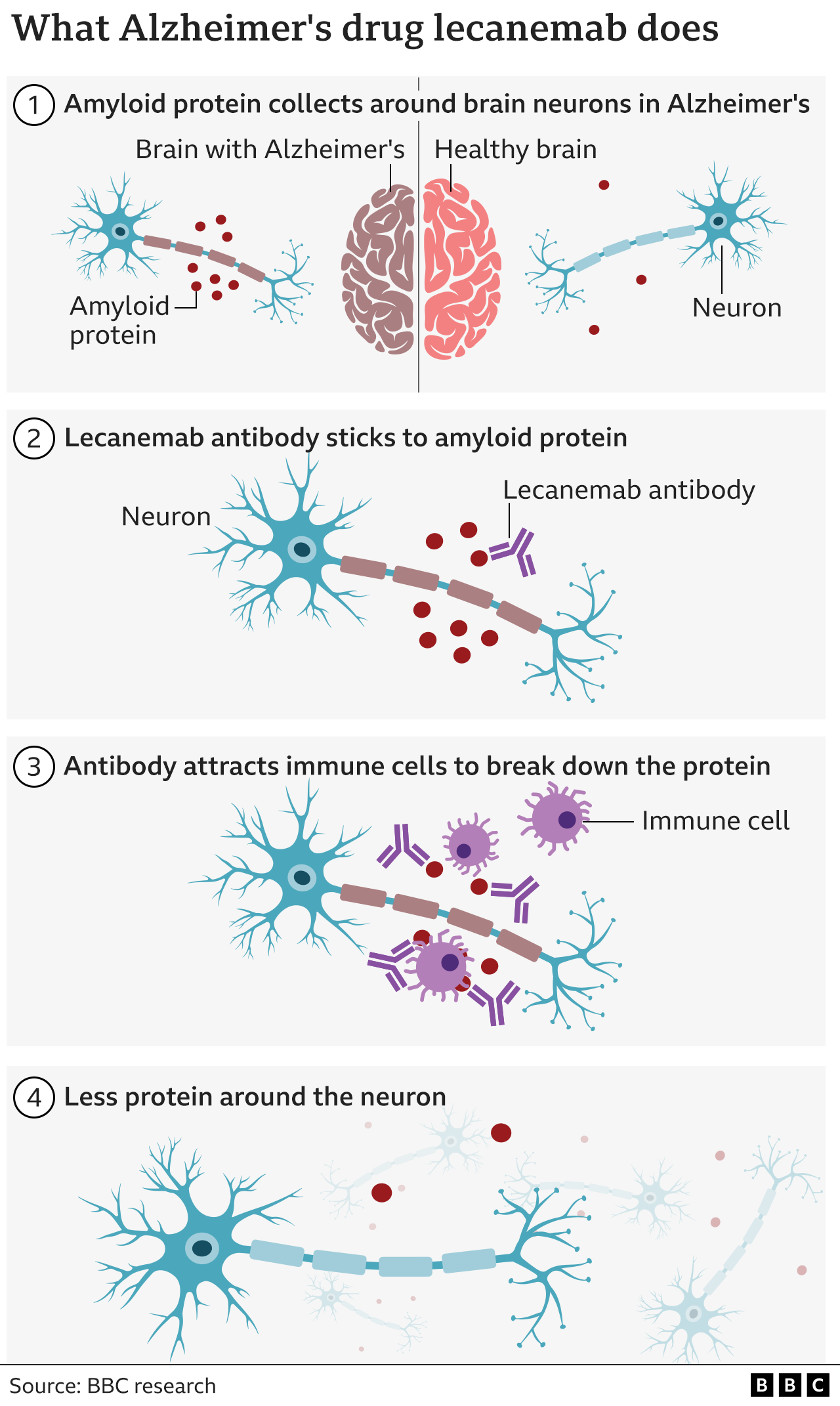
The key finding of the study was that those given lecanemab declined more slowly, relating to a difference of almost half a point on the 18-point scale over the 18 months of the study. The trial's importance lies in its showing that the drug attacks a sticky protein called amyloid, thought to be one of several culprits distinguishing Alzheimer's, and in so doing can delay the progression of the disease, according to Maria Carillo, chief science officer for the Alzheimer's Association.
Yet doctors remain divided over whether that difference is sufficiently significant for patients and their families, particularly since the drug has some serious potential safety risks that include swelling of the brain. "It is unlikely that the small difference reported in this trial will be noticeable to individual patients", was the opinion of Dr. Madhav Thambisetty of the National Institute on Aging, speaking for himself. Many researchers believe a meaningful improvement would reflect at the very least a difference of a full point on that 18-point scale, he pointed out.
Labels: Alzheimer's Disease, New Drug Lecanemab, Pharmaceuticals, Research

0 Comments:
Post a Comment
<< Home