December COVID Vaccine U.S. Rollout
 |
"Filing in the U.S. represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world and we now have a more complete picture of both the efficacy and safety profile of our vaccine.""[Throughout the vaccine development process I wasn't certain Pfizer could produce a safe and effective vaccine ready for FDA review in less than a year.] Conviction is a part of it, so I was always telling [our teams] that we will make it, and we will make it by October, and if not us, then who?:"But I knew that it was an extremely risky suggestion, I knew it was going to be difficult, and the stars needed to be aligned all the way to the end.""[The partnership with BioNTech is built on a mutual focus on advancing science; the two companies began work, including sharing confidential information, before signing a formal contract, since those agreements can take months]. In fact, we are still finalizing the contractual obligations we have that we need to sign with them. It’s the perfect relationship for me.""Ugur [Sahin, BioNTech’s co-founder and chief executive] is a wonderful human being, and a great scientist. He shares the same passion [as I do] about saving lives and I’m very optimistic that not only will we do very well together bringing a COVID-19 vaccine to the world, but later hopefully a flu vaccine."Albert Bouria, chief executive officer, Pfizer Inc.
"[If the data is solid], we literally could be weeks away from the authorization of a 95 percent effective vaccine."U.S.Health Secretary Alex Azar"Within 24 hours from the approval, the vaccine will be moving and located in the areas where each state will have told us where they want the vaccine doses."Dr. Moncef Slaoui, chief scientific adviser, US "Operation Warp Speed" vaccine program
|
Dado Ruvic/Reuters |
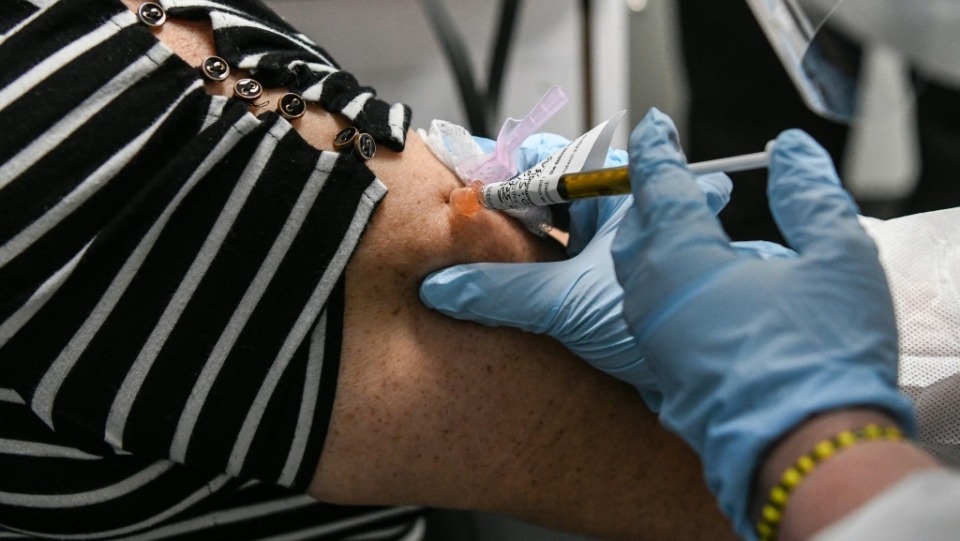
A date has been set for an FDA advisory committee to meet and discuss plans surrounding the COVID vaccine developed by Pfizer and BioNTech. That date is between the 8th and tenth of December. Leading to speculation that as early as December 11 the vaccines could be rolled out and distribution initiated. Pfizer has been busy long before the final Phase Three test results were arrived out, producing the vaccine. It is now prepared to apply to U.S. health regulators for emergency use authorization.
This will be the first of several and perhaps many such applications. Moderna will be certain to follow closely, since the announcement was made public that its COVID-19 vaccine had passed the Third Stage tests with similar results to Pfizer's. AstraZeneca is another contender whose vaccine appears ready for approval, production, distribution and inoculation. These vaccines tested in the amazing 95 percent effective rate with no major safety concerns emerging.
The market responded as affirmatively as the public and government leaders around the globe at the news of the test results, seeing Pfizer's shares rise 1.6 percent and BioNTech's 6 percent. Hopes have been raised for the end of the pandemic responsible for claiming over a quarter of a million lives of people in the United States, and 1-1/3 million lives lost to COVID's complications around the world. Moreover, case numbers are now again steadily rising alarmingly.
 |
| Pfizer's COVID-19 vaccine clinical trial The Associated Press |
Safety data on around 100 children ages between 12 and 15 were included with the application, the company attesting that 45 percent of U.S. trial participants fell between the ages of 56 and 85, both groups representing the demographics hit hardest by the virus. Agreement for the EUA by the FDA is anticipated by mid-December, Pfizer claiming it is prepared to begin shipping doses as soon as agreement is received, expecting to have 50 million doses ready for use this year, which would protect 25 million people in a two-dose protocol.
The Pfizer vaccine provided a level of protection evenly across different ages and ethnic backgrounds, vital news since the virus disproportionately harms the elderly and minority groups. In Pfizer's trial with over 43,000 people participating, there were 170 volunteers who contracted COVID-19, 162 of whom had received a placebo only; the vaccine was adjudged to be 95 percent effective based on those results, eclipsing expectations for a success rate.The FDA had considered a minimum bar for efficacy of 50 percent.
Close to 42 percent of global participants and 30 percent of U.S. participants in the Phase 3 study were of racial and ethnically diverse backgrounds, Pfizer had announced. Next in line at this juncture to seek a U.S. emergency use approval for a COVID-19 vaccine is contender Moderna, and from there the sky's the limit as one health expert after another speaks with a tremor of relief, of "a light at the end of the tunnel".
 |
| FDA vaccine advisors reportedly will meet Dec. 10 to discuss approving vaccines. (AFP) |
Labels: COVID-19, Moderna, Pfizer, United States, Vaccine

0 Comments:
Post a Comment
<< Home