Prioritizing Inoculations
"The government [of Canada] has licensed this drug based on what is known in the clinical trial. So it would be considered a bit untoward to do anything that deviates from the testing procedure in the clinical trial. for example, it wasn't tested on children, so it would be very odd to now go and give it to children.""It probably does work on kids, to be honest, but we won't do it because that's not what we looked at.""So probably it will work on a very high percentage of people who have autoimmune disorders. But we don't know. So it's a precautionary principle at this point, saying that because there's insufficient data, let's hold off, for at least a few months, until we assess the true safety profile."Dr.Raywat Deonandan, epidemiologist, health sciences associate professor, University of Ottawa"At first, I was thinking 'I'm immunosuppressed, and they talked about people who are immunosuppressed, saying they might be at the front of the line. So that's what I thought was going to happen,""I literally don't go out. There's the odd time to get some sanity. But someone' else's cold is my pneumonia.""I don't think I'll feel comfortable going out knowing that 60 or 70 percent of people are [eventually going to be] vaccinated. That leaves at least another 30 percent out there, and I know that I'm at risk to get something.""It would be nice to have more data. But I can wait my turn."Tim Smith, 60, double-lung transplant recipient
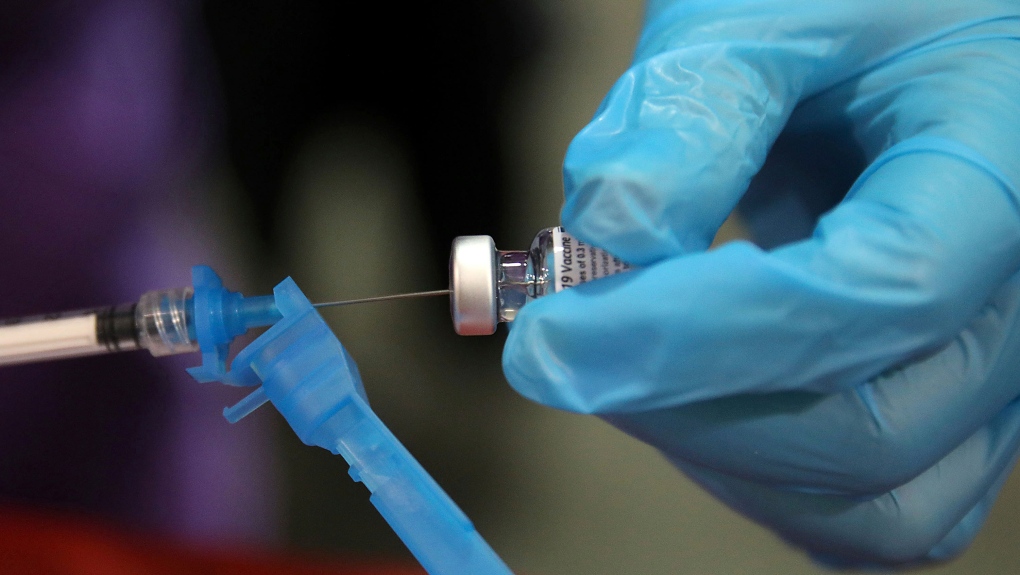 |
| A Pfizer-BioNTech COVID-19 vaccine is prepared prior to being administered, Tuesday, Dec. 15, 2020, in Tulsa, Okla. (Mike Simons/Tulsa World via AP) |
It's a dilemma. Transplant patients and there are plenty of them of all ages in society, face a conundrum. They are among the most needful among the general public of protection from the coronavirus stalking the world community, yet the vaccine trials that have taken place at such lightning speed to attain emergency approval to begin circulating the vaccines -- at the present time in the West, Pfizer and Moderna with more to follow -- did not include immunosuppressed volunteers among the trial subjects, so there is no safety data with respect to that demographic receiving these early inoculations against COVID-19.
A huge disappointment to those people most obviously in need of protection -- as are those with heart conditions, diabetes and respiratory-based illnesses -- who are told they won't be at the front of the line of vaccine recipients after all, they have to be patient and move back, well back, until data is available respecting efficacy and safety specifically reflecting their cases. Both immunologist Dr.Anthony Fauci in the United States, and Ontario's chief medical officer, Dr.Bonnie Henry cautioned that the COVID-19 vaccine is not recommended for people with compromised immune systems.
Canada's National Advisory Committee on Immunization has recommended that the Pfizer-BioNtech vaccine be withheld from people with autoimmune disorders, or people who are immunosuppressed. they also included pregnant women and people under age 16. "It's definitely a disappointment", 30-year-old Danny Norris, another double-lung recipient commented. Both he and is wife who is also a double-lung recipient were looking forward to fortifying their chances of escaping infection by the virus and at the same time being able to resume some semblance of normal life.
"But if everybody else is able to get vaccines, it will help control the spread, which means it will be safer for us in general. Some health officials have said get it, because the vaccine is probably safer than actually catching COVID. No one really knows. But if we're eligible, I'll try to get it as soon as possible", he mused.
 |
| Jeff Kowalsky/AFP via Getty Images |
In the event, high-priority recipients on the list to receive inoculations the soonest as they roll out and become more available to a wider public, start with employees at long-term care homes, and their elderly health-compromised residents. Others are those in the general service industry who are at high risk of exposure, people such as bus drivers and a whole range of essential workers, including medical technicians, nurses and doctors.
Dr.Deonandan points out that there is good news among the doubtful, that the vaccine appears to work effectively on older people; typically they have less responsive immune systems. Additional trials and their resultant evaluations are critical to fully understanding the possible set-backs in expectations. More information is required before the vaccine can be routinely used with special groups like the immunosuppressed. At the very least they will be sheltered by the very fact that others around them are being inoculated, serving to halt the currently-thriving virus.
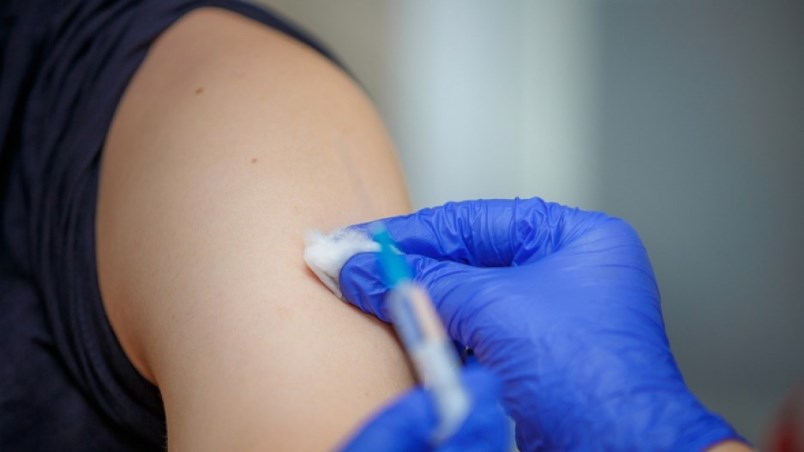 |
| A person gets vaccinated. - Photograph via Getty Images |
Using the measles vaccine as an example, Dr. Wilson points out that it is a live vaccine, unlike the COVID vaccine and so it cannot be administered to the immunocompromised. "That's why everyone else gets vaccinated, to protect those who can't get the vaccine."
"That's where we need to focus.""We call that shielding of the vulnerable, where we vaccinate those who might come in contact with the immunosuppressed."Dr.Kumanan Wilson, physician/scientist, The Ottawa Hospital
Labels: Immunosprressed, Incomplete Trials, Inoculation, Pfizer-BioNTech Vaccine

0 Comments:
Post a Comment
<< Home