"The benefits of the AstraZeneca vaccine against COVID-19 with the associated risks of hospitalization and death, continue to outweigh the risks of the vaccine for the vast majority of people."
"Our safety monitoring systems are working and working effectively, these extremely rare side effects have been identified and analyzed by the best of scientific minds and enabled us to provide scientifically based up-to-date information and advice."
"The balance of benefits and risks of the vaccine is very favourable for older age groups, but it is more finely balanced for younger people, and so we advise that this evidence should be taken into account when considering the use of the vaccine."
Dr.June Raine, chief executive, U.K. Medicines and Healthcare Products Regulatory Agency
 |
| People drink and eat in outdoor street dining areas as
lockdown restrictions are eased amid the coronavirus pandemic in Soho,
London on April 24, 2021. (Toby Melville/Reuters) |
"The U.K. did not have a great supply of the Pfizer vaccine and so, given the decision to vaccinate your population versus a very prolonged vaccine rollout with a pretty significant wave that they had in January 2020 they pushed forward with AstraZeneca."
"They were bold about it, but they should be congratulated on it. They followed the science and they pushed an aggressive campaign to get as many people vaccinated as quickly as possible."
If you were to put everything on a scale and add public good to it, it absolutely would make sense to continue to get as many vaccines to as many people as possible and finish their series."
"But we live in an individual society with individual decision-making and so this is part of the consequences."
Dr.Zain Chagla, infectious disease expert, associate professor, McMaster University, Hamilton, Ontario
 |
A man receives a dose of AstraZeneca’s COVID-19
vaccine at a conference center in Rome on 24 March. Italy halted use of
the vaccine on 15 March, but resumed immunizations 4 days later. Antonio Masiello/Getty Images |
Oxford-AstraZeneca is a vaccine developed in the United Kingdom, so it's hardly surprising, given its fairly high efficacy rate that the U.K. was among the first countries worldwide to approve its use, dispensing millions of doses expeditiously, while the rest of the world was still considering whether to approve the vaccine. In Britain, Pfizer has been utilized half as much, representing the second most-used vaccine in the country.
Developed at Oxford University, the U.K. estimates as of mid-May that 23.9 million doses of AstraZenca were dispensed as a first dose, with nine million second doses. In comparison 11.7 first doses of Pfizer were dispensed, with 9.9 million second doses. Moderna vaccines are only just now beginning to enter Britain, with roughly 200,000 doses being dispersed. The United Kingdom now stands as one of the world's leading countries in vaccinations with at least the first dose having been used to inoculate 56 percent of the population, and fully vaccinating 32 percent of residents.
Britain's view of the rare blood clots associated with AstraZeneca is that of a risk well worth taking in the struggle to contain the SARS-CoV-2 virus causing COVID. The country has been determinedly aggressive in rolling out the vaccine. A situation quite the reverse of what has been happening in Canada. Where the use of AstraZeneca has fallen under a cloak of trepidation over the rare occurrences of vaccine-caused blood clots, despite that COVID-19 is capable of and has indeed caused infinitely greater numbers of blood clots and deaths.
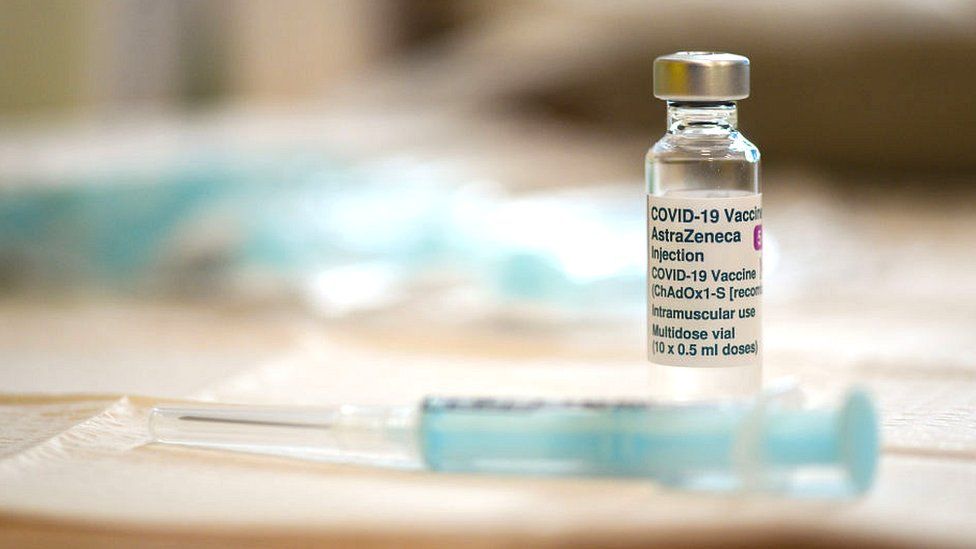 |
Getty Images
|
After temporarily suspending the use of AstraZeneca altogether, the province of Ontario has altered its decision somewhat announcing it would once again use the vaccine, for those people who have already been given their first dose of AstraZeneca, as a follow-up. Other provinces which also had suspended the use of AstraZeneca have not yet decided whether to relax that position.
In the United Kingdom, roughly 250 cases of the rare blood clots associated with the vaccine have occurred. Given the total number of vaccines used, the medical experts place the risk at about one in 100,000 in first doses. Only a handful of clots have arisen in the use of second doses; a rate of one in a million. Percentages that rate similarly in the Ontario experience.
The key difference, according to Dr.Raine, being that the rare, potentially deadly clots are identified in the U.K. medical system, enabling health authorities to treat the clots as they occur. People under 40 who may be at higher risk of acquiring clots are advised to consider waiting for the Pfizer vaccine, while emphasizing that any vaccine is safer than acquiring COVID.
"What we're saying and what we've said all along is that mRNA vaccines are the preferred vaccine", said Dr.Shelley Deeks, co-chair of the National Advisory Council on Immunization in Canada, a volunteer advisory group comprised of health experts, tasked to produce recommendations on vaccine use to the federal government. Their position is that Canadians who can wait for an mRNA vaccine are advised to do so preferentially, bypassing the AstraZeneca vaccine, in an excess of caution.
In early January, Britain was identifying close to 60,000 cases on a daily basis. At the present time, under 3,000 are being presented, with hospital occupancy at its lowest since September. Contrasting with Canada's peaks in early January and in May at around 8,000 cases, with but half the population of the United Kingdom. Britain's 66 million residents recorded over 128,000 COVID deaths, in comparison to Canada's 25,000 deaths among its 36 million population.
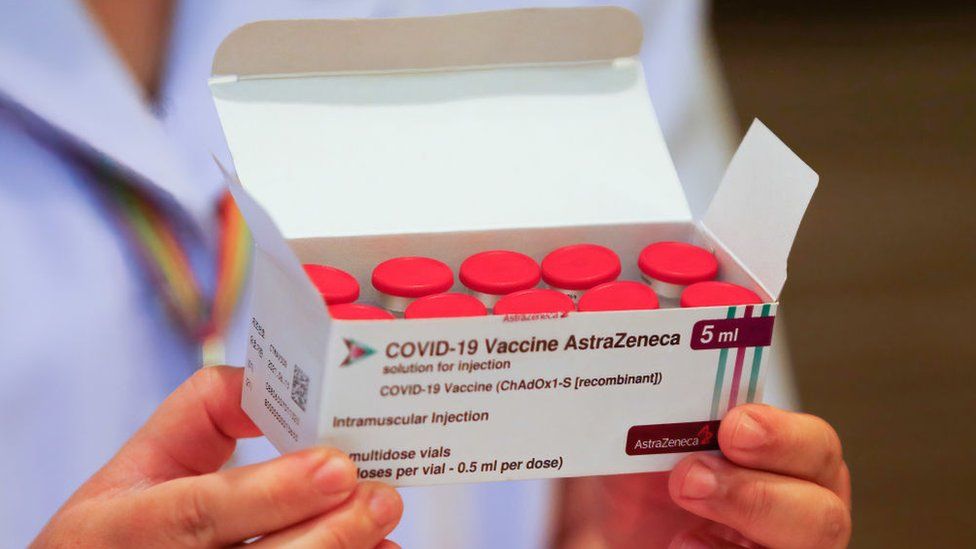 |
Sopa Images
|
Canada is now faced with a moral dilemma. Having advised the population to wait for a mRNA vaccine as represented by Pfizer or Moderna, the country has a stockpile of AstraZeneca vaccines whose expiry date is ticking steadily away; some will expire at the end of May, other stock in June. Canadian provinces must decide whether to proceed with their use during the overall drive to inoculate a large percentage of the population, or to donate them to another country that will intend to use them before they expire.
Countries in desperate need for vaccines would welcome a fresh infusion. "That answer probably needs to come now, especially if doses are expiring at the end of the month You really only have a two-week window to get those doses into people", explained Dr.Chagla.
Labels: Blood Clots, COVID-19 Vaccinations, Global Pandemic, Hesitation, Oxford-AstraZeneca, United Kingdom



 There are experiments taking place in growing algae in low-nutrient environments, in greenhouses as a source of food and the production of oxygen. Survival must include a water source. Mars does contain some sub-surface ice that could be transformed to water. For that purpose the use of radar to map the distribution of sub-surface ice would be invaluable to a future Mars mission. "Once you know where the ice is, those are locations where you might send humans", noted Victoria Hamilton, planetary geologist at the Southwest Research Institute, Boulder, Colorado.
There are experiments taking place in growing algae in low-nutrient environments, in greenhouses as a source of food and the production of oxygen. Survival must include a water source. Mars does contain some sub-surface ice that could be transformed to water. For that purpose the use of radar to map the distribution of sub-surface ice would be invaluable to a future Mars mission. "Once you know where the ice is, those are locations where you might send humans", noted Victoria Hamilton, planetary geologist at the Southwest Research Institute, Boulder, Colorado.














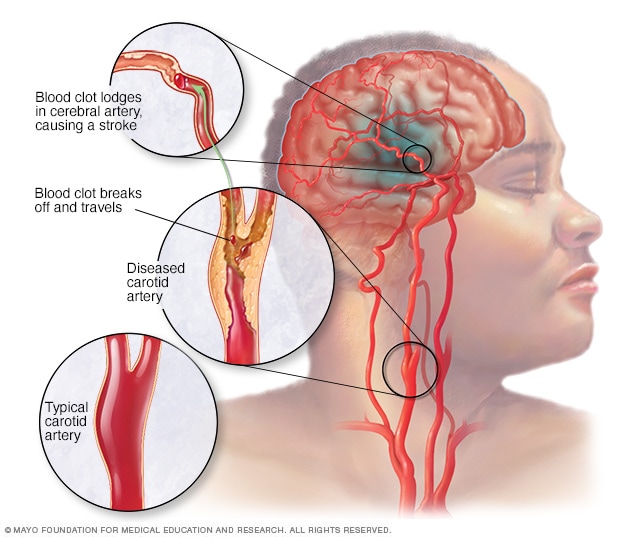 A clot-clearing drug, tissue plasminogen activator (tPA) was approved by Health Canada in 1999 for the treatment of ischemic strokes, but for the drug to be effective, it requires administration within several hours of the onset of stroke symptoms. Since tPA can also be deadly administered to someone who has experienced a hemorrhagic stroke, patients must wait for a CT scan before receiving treatment, a delay whose outcome can be devastating.
A clot-clearing drug, tissue plasminogen activator (tPA) was approved by Health Canada in 1999 for the treatment of ischemic strokes, but for the drug to be effective, it requires administration within several hours of the onset of stroke symptoms. Since tPA can also be deadly administered to someone who has experienced a hemorrhagic stroke, patients must wait for a CT scan before receiving treatment, a delay whose outcome can be devastating. Algernon which harbours the expectation that its microdosing approach may serve to encourage a wider review and acceptance of the therapeutic potential of DMT, introducing the opportunity for further research, believes its approach could have the potential to lead to a Breakthrough Therapy Designation from the FDA, enabling priority review and fast-tracking of the clinical trial process. Dr.Nutt notes that these types of substances require rescheduling for real progress to occur.
Algernon which harbours the expectation that its microdosing approach may serve to encourage a wider review and acceptance of the therapeutic potential of DMT, introducing the opportunity for further research, believes its approach could have the potential to lead to a Breakthrough Therapy Designation from the FDA, enabling priority review and fast-tracking of the clinical trial process. Dr.Nutt notes that these types of substances require rescheduling for real progress to occur.